Chapter 14: Chirality - Handedness of Molecules
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
43 Terms
Isomers
same molecular formula but different connectivity
subdivided into: stereoisomers and constitutional isomers
Stereoisomers
same connectivity
subdivided into: achiral and chiral
Constitutional isomers
different connectivity
Achiral
without stereocenters
cis-trans isomers
Chiral
with stereocenters
subdivided into: enantiomers and diastereomers
Diastereomers
superposable mirror images
Enantiomers
non superposable mirror images
ex: 2-butanol
always come in pairs
Rotate mirror image
one way to see that the mirror image of molecule is not superposable on the original molecule
Chiral
objects that are non superposable on their mirror images
greek: cheir - hand
they show handedness
Presence of carbon bonded with 4 different groups
most common cause of enantiomerism in organic molecules
Stereocenter
carbon with 4 different groups bonded to it
Achiral
An object and its mirror image are superposable, they are identical and there is no possibility of enantiomerism
ex: 2-propanol (has no stereocenter)
180 degrees
degree of rotation to see that the mirror image of 2-butanol is not superposable on the original
120 degrees
Amount of degrees to rotate the mirror image to see its relationship with the original molecule
it turns the mirror image back to how the original looked like
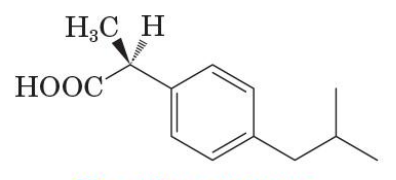
Inactive enantiomer of ibuprofen
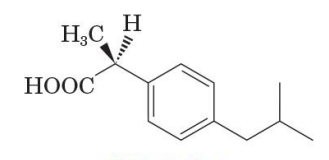
active enantiomer of ibuprofen
R,S system
way to distinguish between enantiomers without having to draw them and point to one or the other
First step in assigning an R or S configuration to stereocenter
arrange the groups on the stereocenter in order of priority (higher atomic number, higher priority)
To assign an R or S configuration
Assign a priority from 1 (highest) to 4 (lowest) to each group bonded to the stereocenter
Orient the molecule in space so that the group of lowest priority (4) is directed away from you. The three groups of higher priority (1–3) then project toward you
Read the three groups projecting toward you in order from highest (1) to lowest (3) priority
If reading the groups 1-2-3 is clockwise, the configuration is R. If reading them is counterclockwise, the configuration is S
R configuration
If reading the (highest) groups 1-2-3 is clockwise
S configuration
if reading the (highest) groups 1-2-3 is counterclockwise
2^n
for molecules with n stereocenters, maximum number of possible stereoisomers
Molecule with 1 stereocenter
2^1 = 2 stereoisomers (one pair of enantiomers) are possible
Molecule with 2 stereocenters
maximum of 2^2 = 4 stereoisomers (two pairs of enantiomers) are possible
Molecule with 3 stereocenters
maximum of 2³ = 8 stereoisomers (four pairs of enantiomers) are possible
Ordinary light
light waves oscillating in all planes perpendicular to its direction of propagation
Plane-polarized light
light waves oscillating only in parallel planes
Polarimeter
an instrument for measuring the ability of a compound to rotate the plane of plane-polarized light
Optically active
showing that a compound is capable of rotating the plane of plane-polarized light
Dextrorotatory
Clockwise rotation of the plane of plane-polarized light
Indicated by (+)
Levorotatory
Counterclockwise rotation of the plane of plane-polarized light
Indicated by (–)
Specific rotation
observed rotation of an optically active substance at a concentration of 1 g/mL in a sample tube 10 cm long
Molecules in living systems (plant and animals)
are chiral except for inorganic salts and a few low-molecular-weight organic substances
One stereoisomer
amount of stereoisomer found in nature
More than 1 stereoisomer in nature
possible but rarely exist together in the same biological system
(R)-glyceraldehyde
fits the 3 binding sites on enzyme surface
(S)-glyceraldehyde
fits only 2 of the 3 binding sites on enzyme surface
Enzymes (protein biocatalysts)
all have many stereocenters
ex: chymotrypsin
because they are chiral substances, most either produce or react with only substances that match their stereochemical requirements
Chymotrypsin
251 stereoisomers
2^251!
maximum number of possible stereoisomers
only one of these stereoisomers is produced and used by any given organism
Chiral environment
interactions between molecules in living systems take place here
this causes a molecule and its enantiomer or one of its diastereomers elicit different physiological responses
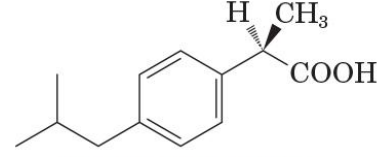
(S)-ibuprofen
active as pain and fever reliever
its R enantiomer is inactive
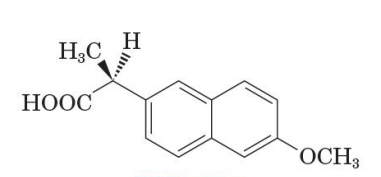
S enantiomer of naproxen
active pain reliver
R enantiomer is a liver toxin