Biological molecules
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
Common protection methods for N-terminus of an amino acid (general) (2)
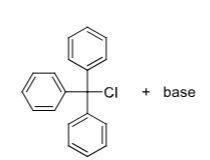
Boc (Boc2O) + base, Fmoc-Cl + base
How to deprotect Boc
TFA F3C-COOH
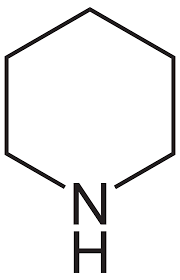
How to deprotect Fmoc
piperidine
Common protecting groups of the carboxylic acid of the C-terminus (general) (4)
generally esterification with an alcohol (MeOH + HCl(methyl ester)/ Bn-COH+ HCl(benzyl ester)/ allyl ester/ tert-butyl ester)
How to deprotect the methyl ester
NaOH, H2O
how to deprotect the benzyl ester/ether
H2/ Pd/C
how to deprotect the allyl ester
Pd(PPh3)4
how to deprotect tert-butyl ester/ether?
TFA
common methods to protect hydroxyl groups (3)
tert-butyl ether, benzyl ether, silyl ether
How to deprotect a silyl ether
TBAF
Whats the issue with activating the C-terminus as an acid chloride
racemisation of the activated amino acid forming unwanted byproducts with the wrong stereochemistry caused by oxazolone formation
what do we use to activate the C- terminus and examples (2)
coupling reagent, DCC and HATU +base
How to selectively protect the anomeric OH
MeOH + HCl
How to selectively deprotect the anomeric OH?
H2NNH2 or benzyl amine
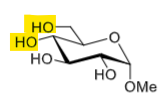
How to selectively protect the 1,3-diols?
Benzylidene acetal + acid
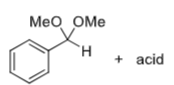
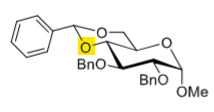
How to selectively deprotect the C4-OH
NaBH4
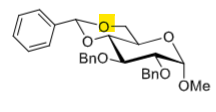
How to selectively deprotect the C6-OH
LiAlH4
Which coupling reagent should be used during SPPS and why?
DIC because DCC produces an insoluble urea byproduct
What do we generally use to protect the N-terminus for SPPS and why?
Fmoc, Boc uses acidic conditions to deprotect which would release the peptide from the resin.
What can we use to activate the anomeric hydroxyl group in glycosidic bond formation?
HBr (followed by Ag2CO3 and AgOTf), CCl3CN + NaH (activated by TMSOTf), PhSH + BF3 (followed by further activation)
What often leads to the major product of glycosidic bond formation being alpha?
anomeric effect
What effect leads to the formation of the 1,2-trans isomer in glycosidic bond formation?
neighbouring group participation, usually a ester protecting group
what is intramolecular tethering used for the formation of?
1,2-cis glycosidic bonds
What is commonly used for intramolecular tethering?
Me2SiCl2
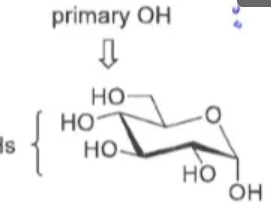
How to selectively protect a primary OH of a sugar?
Triphenyl methyl ether (Trityl)