Materials Science I
1/305
Earn XP
Description and Tags
Hell on earth.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
306 Terms
The evolution of engineering materials
Engineering materials evolved from naturally occurring materials such as stone, wood, and clay to metals, polymers, ceramics, composites, electronic materials, biomaterials, and nanomaterials as human needs, technology, and processing methods advanced.
Why new classes of materials were developed
New materials were developed to meet increasing demands for higher strength, lower weight, better thermal and electrical performance, corrosion resistance, durability, and functionality.
Key stages in the evolution of materials
Stone Age (natural materials), Bronze and Iron Ages (metals), Industrial Age (steels and alloys), Polymer Age (synthetic polymers), and Modern Age (composites, semiconductors, biomaterials, nanomaterials).
Material properties
Material properties describe how a material responds to mechanical, thermal, electrical, magnetic, optical, and chemical influences.
Design-limiting properties
Design-limiting properties are material properties that must meet minimum required values for a component to function safely and effectively.
Examples of design-limiting properties
Stiffness, strength, toughness, electrical resistivity, thermal conductivity, and optical quality.
Why design-limiting properties are important
If any design-limiting property is inadequate, the component will fail regardless of how good other properties are.
Mechanical properties
Mechanical properties describe a material’s response to applied forces or loads.
Key mechanical properties
Stiffness, strength, hardness, ductility, toughness, and resistance to fracture.
Why mechanical properties matter in design
They determine whether a material can support loads, deform safely, and resist failure during service.
Thermal properties
Thermal properties describe how materials respond to temperature changes and heat flow.
Thermal conductivity
Thermal conductivity is the ability of a material to conduct heat.
Thermal diffusivity
Thermal diffusivity measures how quickly heat spreads through a material and is proportional to thermal conductivity divided by heat capacity.
Importance of thermal diffusivity
It determines how fast a material heats up or cools down for a given thickness.
Electrical conductivity
Electrical conductivity describes how easily electric current flows through a material.
Electrical resistivity
Electrical resistivity is the inverse of electrical conductivity and measures resistance to current flow.
Magnetic properties
Magnetic properties describe how materials respond to magnetic fields.
Hard magnetic materials
Hard magnetic materials retain magnetization and are used as permanent magnets.
Soft magnetic materials
Soft magnetic materials are easily magnetized and demagnetized and are used in transformers and electric motors.
Chemical properties
Chemical properties describe a material’s resistance to chemical attack and corrosion.
Common aggressive environments
Water, salt water, acids, alkalis, organic solvents, oxidizing flames, and ultraviolet radiation.
Optical properties
Optical properties describe how materials interact with light through reflection, refraction, absorption, or transmission.
Examples of optical behavior
Opaque materials reflect light, transparent materials refract light, and some materials selectively absorb wavelengths.
Density
Density is the mass per unit volume of a material.
Density trends among materials
Metals generally have high densities, polymers have low densities, and ceramics have intermediate densities.
Why density matters
Density affects weight, structural efficiency, and suitability for lightweight or load-bearing applications.
Stiffness
Stiffness is the resistance of a material to elastic deformation and is measured by the elastic modulus.
Stiffness comparison
Ceramics and metals are generally stiffer than polymers.
Why stiffness is important
High stiffness is required when dimensional stability under load is critical.
Strength
Strength is the ability of a material to withstand applied stress without failure.
Strength comparison
Metals and ceramics generally have higher strengths than polymers.
Role of temperature
Strength values are usually specified at room temperature unless otherwise stated.
Resistance to fracture
Resistance to fracture describes a material’s ability to resist crack initiation and propagation.
Brittle vs ductile behavior
Ceramics are typically brittle with low fracture resistance, while metals are more ductile with higher fracture resistance.
Importance of temperature resistance to fracture in design
Low fracture resistance can lead to sudden and catastrophic failure.
Conductivity comparison
Metals have high electrical conductivity, polymers and ceramics usually have very low conductivity, and semiconductors have intermediate conductivity.
Importance of electrical conductivity
Electrical conductivity is a design-limiting property in electrical and electronic applications.
Metallic materials
Metallic materials are characterized by metallic bonding, high electrical and thermal conductivity, and good ductility.
Typical properties of metals
High strength, good toughness, high density, and good formability.
Examples of metallic materials
Steel, aluminum, copper, titanium, and their alloys.
Polymeric materials
Polymeric materials are composed of long-chain molecules made of repeating units called mers.
Typical properties of polymers
Low density, low stiffness and strength, good corrosion resistance, and low thermal and electrical conductivity.
Examples of polymers
Polyethylene, polypropylene, polyvinyl chloride, polystyrene, and nylon.
Ceramic materials
Ceramics are inorganic, non-metallic materials composed of metallic and non-metallic elements.
Mechanical behavior of ceramics
Ceramics are stiff and hard, strong in compression, weak in tension, and brittle.
Other properties of ceramics
Low electrical and thermal conductivity, high temperature resistance, and good chemical stability.
Examples of ceramic materials
Glass, porcelain, bricks, concrete, alumina, and silicon carbide.
Composite materials
Composites are materials composed of two or more distinct phases combined to achieve superior properties.
Matrix and reinforcement
One phase acts as a matrix and the other as a reinforcing phase.
Types of composites
Metal-matrix, ceramic-matrix, and polymer-matrix composites.
Purpose of composites
To combine the best properties of different materials and achieve synergistic effects.
Electronic materials
Electronic materials are materials used for their electrical or electronic functionality, especially semiconducting behavior.
Key electronic materials
Silicon, germanium, gallium arsenide.
Examples of use of electronic materials
Integrated circuits, optical fibers, interconnects, and dielectric layers.
Biomaterials
Biomaterials are natural or synthetic materials designed to interact with biological systems.
Key requirement of biomaterials
Biocompatibility, meaning they do not cause adverse reactions in the human body.
Examples of biomaterials
Metals, ceramics, polymers, and composites used in implants, prostheses, and medical devices.
Nanomaterials
Nanomaterials are materials that have at least one structural dimension in the nanoscale range (1
Classification of materials by dimensionality
0D (nanoparticles), 1D (nanowires, nanotubes), 2D (nanofilms, nanocoatings), and 3D (bulk nanostructured materials).
Why nanomaterials are special
They often exhibit unique mechanical, electrical, optical, and chemical properties due to their small size.
Internal structure of materials
The internal structure refers to the arrangement of atoms, ions, or molecules within a material.
Levels of structure
Atomic structure, crystal structure, microstructure, and macrostructure.
Why internal structure matters
Internal structure determines material properties and performance.
Atomic structure
Atomic structure describes the organization of protons and neutrons in the nucleus and electrons surrounding the nucleus.
Key atomic particles
Protons (positive), neutrons (neutral), and electrons (negative).
Importance of atomic structure
It determines chemical behavior and bonding characteristics.
Electrons in atoms
Electrons are negatively charged particles that occupy discrete energy levels or shells around the nucleus.
Role of electrons
Electrons determine chemical properties and bonding behavior of atoms.
Energy levels and shells
Electrons occupy shells labeled K, L, M, N…, corresponding to increasing energy and distance from the nucleus.
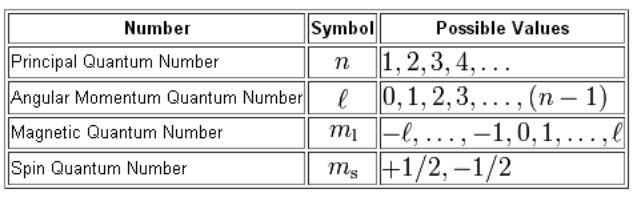
Quantum numbers
Quantum numbers describe the unique quantum state of an electron in an atom.
The four quantum numbers
Principal (n), azimuthal (l), magnetic (ml), and spin (ms).
Meaning of each quantum number
n (principal) defines the energy level or shell,
l (azimuthal) defines the subshell or orbital shape (s, p, d, f),
m_l (magnetic) defines the orientation of the orbital,
m_s (spin) defines the electron’s spin direction (+½ or −½).
Electron configuration
Electron configuration shows the arrangement of electrons in an atom’s shells and orbitals.
Rules for electron configuration
Aufbau principle (fill lowest energy orbitals first), Pauli exclusion principle (no two electrons have the same set of quantum numbers), Hund’s rule (maximize unpaired electrons in degenerate orbitals).
Periodic table
A tabular arrangement of elements ordered by atomic number, showing recurring (“periodic”) chemical properties.
Groups and periods
Groups (columns) share similar chemical behavior, periods (rows) represent increasing principal energy levels.
Why the periodic table is important
It allows prediction of element properties, reactivity, and trends such as atomic radius, ionization energy, and electronegativity.
Atomic bonding
Atomic bonding is the interaction that holds atoms together in a material.
Types of bonding
Ionic, covalent, metallic, and van der Waals (secondary) bonding.
Effect of bonding on material properties
Bond type determines mechanical, thermal, electrical, and chemical properties of solids.
Bonding forces
Forces that hold atoms together include electrostatic attraction (ionic), shared electron pairs (covalent), delocalized electrons (metallic), and weak dipole interactions (van der Waals).
Bonding energy
Energy required to break a bond; higher bonding energy usually results in higher melting and boiling points.
Primary bonds
Strong bonds that involve the transfer or sharing of electrons.
Types of interatomic bonds
Ionic bonds: electron transfer between atoms (e.g., NaCl),
Covalent bonds: electron sharing between atoms (e.g., diamond),
Metallic bonds: delocalized electrons shared across many atoms (e.g., copper).
Ionic bonding
Occurs between atoms with large differences in electronegativity (metal + nonmetal).
Covalent bonding
Occurs between atoms with similar electronegativities (usually nonmetals).
Bonding character
Most bonds are not purely ionic or covalent but have a degree of both.
Metallic bonding
Characterized by delocalized electrons moving freely among positive ion cores.
Properties of metallic bonding
High electrical and thermal conductivity, ductility, malleability, and shiny appearance.
Secondary bonding
Weak attractive forces between atoms or molecules that are not due to the sharing or transfer of electrons, unlike primary (ionic, covalent, metallic) bonds.
Types of secondary bonding
London dispersion forces (temporary dipoles), dipole-dipole interactions, and hydrogen bonding.
Effect of secondary bonding
Secondary bonds are weaker than primary bonds and influence properties such as melting point, boiling point, and solubility.
Quantum mechanics
Describes the behavior of electrons in atoms using wave functions.
Atomic orbitals
Regions of space where electrons are most likely to be found. Atomic orbitals explain the distribution of electrons, chemical bonding, and material properties.
Orbitals
Regions of space around an atom’s nucleus where there is a high probability of finding an electron. Defined by quantum numbers (n, l, ml, ms).
Shapes of orbitals
s: spherical,
p: dumbbell-shaped,
d and f: more complex shapes.
Each orbital can hold a maximum of two electrons with opposite spins.
Contour representation
Uses lines or surfaces to represent regions of constant electron probability. Visualizes orbital shapes and electron density distributions in space.
p orbitals
Three degenerate orbitals (px, py, pz) oriented along x, y, z axes. Dumbbell-shaped, each holds two electrons, important in covalent bonding.
d orbitals
Five orbitals with complex cloverleaf shapes, important in transition metals.
f orbitals
Seven orbitals with highly complex shapes, significant in lanthanides and actinides.