Module 5 - Physical Chemistry and Transition Elements
1/101
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
102 Terms
[5.1.3] What is a Brønsted–Lowry acid?
A substance that donates a proton.
[5.1.3] What is a Brønsted–Lowry base?
A substance that accepts a proton.
[5.1.3] What are conjugate acid-base pairs?
Conjugate acid-base pairs contain two species that can be interconverted by the transfer of a proton.
e.g. in the dissociation of hydrochloric acid:
HCl ⇌ H⁺ + Cl⁻
In the forward reaction, HCl releases a proton to form its conjugate base, Cl⁻.
In the reverse reaction, Cl⁻ accepts a proton to form its conjugate acid, HCl.
[5.1.3] What is an example of conjugate base pairing?
C₆H₅COOH (pKₐ = 4.19), CH₃CHOHCOOH (pKₐ = 3.86)
C₆H₅COOH (B1) + CH₃CHOHCOOH (A1) ⇌ C₆H₅COOH₂⁺ (A1) + CH₃CHOHCOO⁻ (B2)
the stronger acid donates a proton
[5.1.3] What is a monobasic acid?
An acid with one hydrogen present, e.g. HCl.
[5.1.3] What is a dibasic acid?
An acid with two hydrogens present, e.g. H₂SO₄.
[5.1.3] What is a tribasic acid?
An acid with three hydrogens present, e.g. H₃PO₄.
[5.1.3] Which equation is used to determine the pH of a substance?
pH = -log[H⁺]
[5.1.3] Which equation is used to determine the [H⁺] from its pH?
[H⁺] = 10⁻ᵖᴴ
[5.1.3] What do we assume about strong acids?
We assume that [H⁺] = [HA] as strong acids fully dissociate. The exception is dibasic acids.
[5.1.3] What do we assume about weak acids?
We cannot assume that [H⁺] = [HA] as weak acids only partially dissociate. However, [H⁺] = [A⁻] as each [HA] will dissociate into [H⁺][A⁻].
[5.1.3] What else do we assume about [HA]?
We assume that [HA] at equilibrium is the same as the initial concentration.
[5.1.3] What is the acid dissociation constant?
Kₐ = [H⁺][A⁻] / [HA]
[5.1.3] How can the Kₐ equation be mathematically simplified?
Kₐ = [H⁺]² / [HA]
[5.1.3] How is Kₐ an indicator of acid strength?
Stronger acids have a higher Kₐ value.
[5.1.3] What is the link between Kₐ and pKₐ?
Kₐ and pKₐ both show the strength of an acid.
[5.1.3] What is the equation for pKₐ?
pKₐ = -logKₐ
[5.1.3] How can pKₐ be converted back to Kₐ?
Kₐ = 10⁻ᵖᴷᵃ
[5.1.3] How is pKₐ an indicator of acid strength?
Lower pKₐ = stronger acid.
[5.1.3] What is a strong base?
A substance that accepts protons, and fully dissociates to release all its OH⁻ ions.
[5.1.3] What is the ionic product of water?
The mathematical product of the concentration of hydrogen ions and hydroxide ions.
[5.1.3] What is the Kw equation?
Kw = [H⁺][OH⁻]
[5.1.3] What is the value of Kw at room temperature?
1 × 10⁻¹⁴mol²dm⁻⁶
[5.1.3] Water has a different pH at its boiling point, but remains neutral. Why?
pH decreases as the temperature increases, the equilibrium shifts to favour the forward reaction (endothermic side) and the [H⁺] increases.
Water remains neutral as [H⁺] = [OH⁻].
[5.1.3] What do we assume about strong bases?
We cannot assume [H⁺] = [OH⁻] as we have extra OH⁻ ions; we use the base concentration as the [OH⁻] concentration.
[H⁺] = Kw / [OH⁻]
[5.1.3] What are conjugate acid-base pairs?
Two species that can be interconverted by the transfer of a proton.
[5.1.3] In the equation: NH₃ + H₂O ⇌ NH₄⁺ + OH⁻, what is the conjugate acid and conjugate base?
Base (B1) = NH₃
Conjugate Base (B2) = OH⁻
Acid (A1) = H₂O
Conjugate Acid = NH₄⁺
[5.1.3] In a reaction between two acids, how do we work out the conjugate acid and conjugate base?
The stronger acid is the proton donor, and the weaker acid is the proton acceptor.
[5.1.3] What is a buffer?
Systems which minimise pH changes on addition of small amounts of acid or base.
[5.1.3] What is a weak acid buffer?
A weak acid and a salt of the weak acid, dissolved in water.
[5.1.3] What assumption do we make for buffer calculations?
[H⁺] = Kₐ x [HA]/[A⁻]
[5.1.3] How does equilibrium affect buffers?
HA ⇌ H⁺ + A⁻
Adding acid will increase [H⁺] so the equilibrium shifts to the left.
Adding base will shift equilibrium to the right as H⁺ reacts with OH⁻ to make water, causing [H⁺] to decrease.
\
It is used as a measure of **ionic bond strength**.
[5.2.3] What is meant by the term ‘oxidising agent?
A species that oxidises another species, and is itself reduced.
[5.2.3] What is meant by the term ‘reducing agent?
A species that reduces another species, and is itself oxidised.
[5.2.3] What are the half-equation rules?
Balance all atoms that are not O or H.
Balance O by adding H₂O.
Balance H by adding H⁺.
Balance Charge by adding e⁻.
Add State Symbols.
[5.2.3] What is meant by the standard electrode potential of a cell?
The E° of a half-cell is the electromotive force (e.m.f) of a half cell compared with a standard hydrogen half cell measured at standard conditions.
The more negative the E°, the larger the tendency for the half cell to be oxidised (to release electrons).
[5.2.3] What value are standard hydrogen electrodes assigned?
0.00v
[5.2.3] What does a standard hydrogen electrode look like?
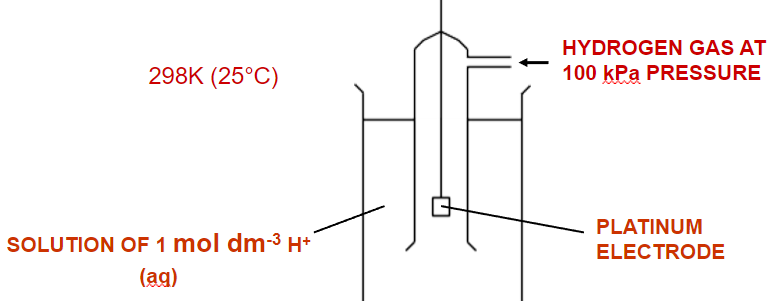
[5.2.3] Why are platinum electrodes typically used?
They are inert and conductive.
[5.2.3] What are the standard conditions for electrode potentials?
298K, 100kPa, 1.00moldm⁻³.
[5.2.3] What does an electrochemical cell look like?
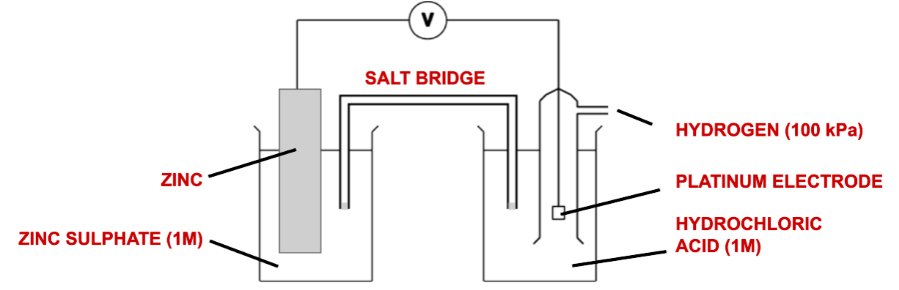
[5.2.3] What are the three types of half cell?
Metals in contact with solutions of their ions.
Gases in contact with solutions of their ions.
Solutions of Ions in two different oxidation states.
[5.2.3] How can the cell potential be calculated?
Positive Value - Negative Value
[5.2.3] What are the three types of cell?
Non-Rechargeable Storage Cell
Rechargeable Storage Cell
Fuel Cell
[5.2.3] What is a non-rechargeable storage cell?
A cell that is discarded once the voltage falls.
[5.2.3] What is a rechargeable storage cell?
A cell whereby the reaction is reversed, and its chemicals are regenerated.
[5.2.3] What is a fuel cell?
A cell that generates electrical energy using external supplies of a fuel and oxidising agent.
[5.2.3] What are fuel cells made up of?
Anode, Cathode, Electrolytes.
[5.2.3] What is the net result of the occurring reactions in a fuel cell?
Fuel reacts with oxygen.
Water is produced.
Voltage is produced.
[5.2.3] What does a typical hydrogen-oxygen fuel cell look like?
This specific example uses an acidic electrolyte.
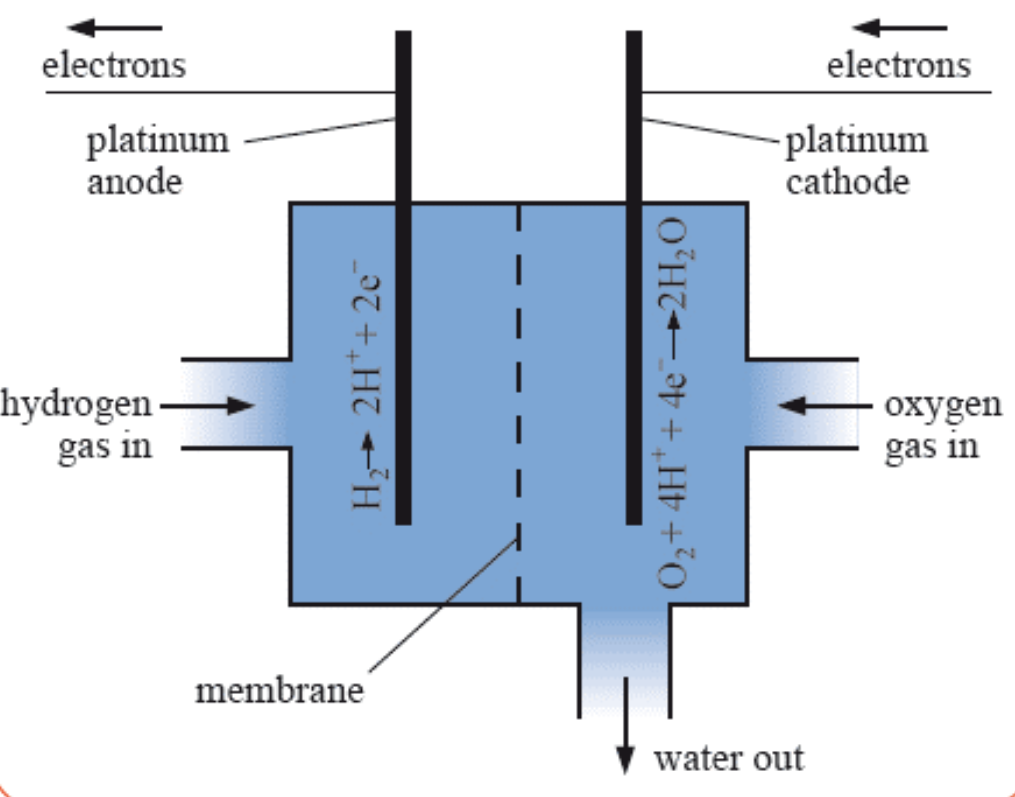
[5.2.3] What is an alkaline hydrogen-oxygen fuel cell?
Alkaline fuel cells use alkaline electrolytes and the membrane only allows for the movement of hydroxide ions.
[5.3.1] What are transition metals?
D-block elements that form at least one ion with an incomplete d-subshell.
[5.3.1]
[5.3.1] What shape is formed by [Fe(NH₃)₄(H₂O)₂]²⁺?
Octahedral (bond angle of 90 degrees).
[5.3.1] What shape is formed by [CuCl₄]²⁻?
Tetrahedral (bond angle of 109.5 degrees).
[5.3.1] Why are tetrahedral shapes formed by transition metals?
When the ligands are larger.
[5.3.1] Which metals form square planar molecules?
Group 10 Molecules, e.g. Ni/Pt
[5.3.1] What shape is formed by AgCl₂?
Linear (bond angle of 180 degrees).
[5.3.1] What are the cis/trans isomers of [Pd(NH₃)₂Cl₂]?
In the cis-isomer, the bond angle is 90°.
In the trans-isomer, the bond angle is 180°.
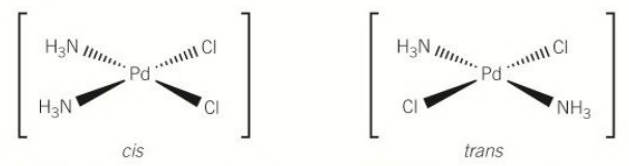
[5.3.1] What is the structure of cis-platin?
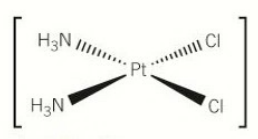
[5.3.1] How does cis-platin work as an anti-cancer drug?
Loses chloride ligands.
Binds to DNA, causing it to bend.
Inhibits DNA synthesis.
[5.3.1] How does [Cu(NH₂CH₂CH₂NH₂)₂(H₂O)₂]²⁺ present cis/trans isomerism?
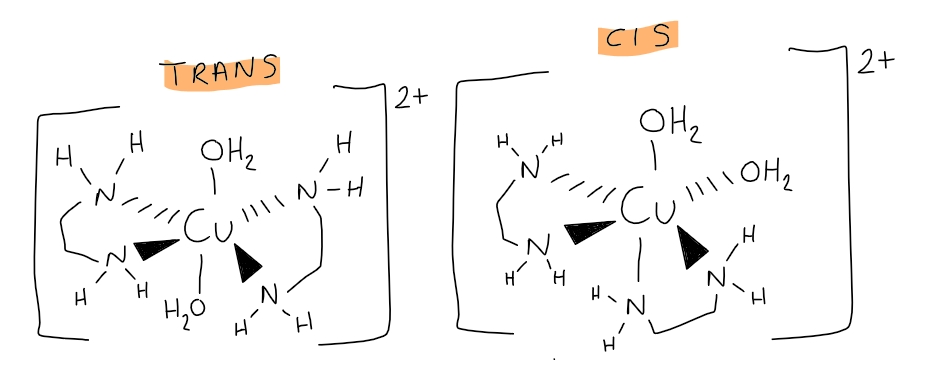
[5.3.1] How does cis-[Cu(NH₂CH₂CH₂NH₂)₂(H₂O)₂]²⁺ present optical isomerism?
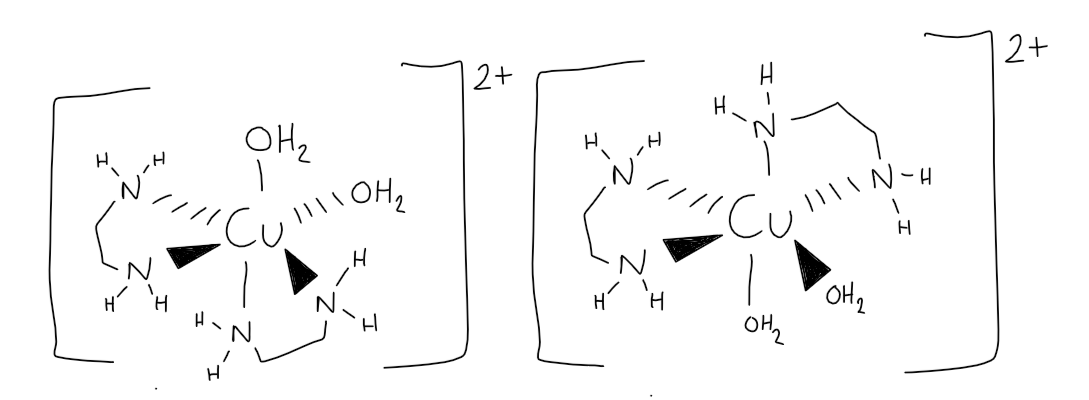
[5.3.1] Why are carbons present in bidentate ligands?
In order to allow them to ‘stretch’ to bond to two parts of the octahedral shape.
[5.3.1] What are ligand substitution reactions?
A reaction in which one ligand is replaced by another.
[5.3.1] How is [Cu(NH₃)₄(H₂O)₂]²⁺ formed from [Cu(H₂O)₆]²⁺?
[Cu(H₂O)₆]²⁺ + 4NH₃ ⇌ [Cu(NH₃)₄(H₂O)₂]²⁺ + 4H₂O
[5.3.1] What is the colour change associated with the formation of [Cu(NH₃)₄(H₂O)₂]²⁺ from [Cu(H₂O)₆]²⁺?
Blue —> Dark Blue
[5.3.1] How is [CuCl₄]²⁻ formed from [Cu(H₂O)₆]²⁺?
[Cu(H₂O)₆]²⁺ + 4HCl ⇌ [CuCl₄]²⁻ + 6H₂O + 4H⁺
[5.3.1] What is the colour change associated with the formation of [CuCl₄]²⁻ from [Cu(H₂O)₆]²⁺?
[CuCl₄]²⁻ is a yellow product.
The resulting solution is green as there is an equilibrium between [Cu(H₂O)₆]²⁺ (blue) and [CuCl₄]²⁻ (yellow).
[5.3.1] How is [Cr(NH₃)₆]³⁺ formed from [Cr(H₂O)₆]³⁺?
[Cr(H₂O)₆]³⁺ + 6NH₃ ⇌ [Cr(NH₃)₆]³⁺ + 6H₂O
[5.3.1] What is the colour change associated with the formation of [Cr(NH₃)₆]³⁺ from [Cr(H₂O)₆]³⁺?
Violet —> Purple
[5.3.1] Why is Fe²⁺ important in haemoglobin?
Binds to oxygen to form oxyhaemoglobin, in order to transport it around the body.
[5.3.1] Why is the ligand substitution in Fe²⁺ of oxygen to carbon monoxide bad?
CO binds to Hb more strongly than O₂, which prevents O₂ being transported around the body.
The equilibrium makes it nearly impossible for CO to be substituted for another ligand, meaning that if carboxyhaemoglobin concentration becomes too high, death can occur.
[5.3.1] What is a precipitation reaction?
When two aqueous solutions containing ions react together to produce a precipitate.
[5.3.1] How is Cu(OH₂) formed from [Cu(H₂O)₆]²⁺?
Cu²⁺₍ₐᵩ₎ + 2NaOH₍ₐᵩ₎ —> Cu(OH)₂₍ₛ₎
Ionic Equation = [Cu(H₂O)₆]²⁺₍ₐᵩ₎ + 2OH⁻₍ₐᵩ₎ —> [Cu(H₂O)₄(OH)₂]₍ₛ₎ + 2H₂O₍ₗ₎
[5.3.1] What is the colour change in the formation of Cu(OH)₂ from Cu²⁺?
Blue —> Light Blue
[5.3.1] How is Fe(OH)₂ formed from Fe²⁺?
Fe²⁺₍ₐᵩ₎ + 2OH⁻₍ₐᵩ₎ —> Fe(OH)₂₍ₛ₎
[5.3.1] What is the colour change in the formation of Fe(OH)₂ from Fe²⁺?
Pale Green —> Dark Green
[5.3.1] How is Fe(OH)₃ formed from Fe³⁺?
Fe³⁺₍ₐᵩ₎ + 3OH⁻₍ₐᵩ₎ —> Fe(OH)₃₍ₛ₎
[5.3.1] What is the colour change in the formation of Fe(OH)₃ from Fe³⁺?
Yellow —> Red/Brown
[5.3.1] How is Cr(OH)₃ formed from Cr³⁺?
Cr³⁺₍ₐᵩ₎ + 3OH⁻₍ₐᵩ₎ —> Cr(OH)₃₍ₛ₎
[5.3.1] What is the colour change in the formation of Cr(OH)₃ from Cr³⁺?
Purple —> Green/Grey
[5.3.1] How is [Cr(OH)₆]³⁻ formed from [Cr(H₂O)₆]³⁺?
[Cr(H₂O)₆]³₍ₐᵩ₎+ 6OH⁻₍ₐᵩ₎ —> [Cr(H₂O)₆]³⁺₍ₛ₎ + 6H₂O
[5.3.1] What is the colour change in the formation of [Cr(OH)₆]³⁻ from [Cr(H₂O)₆]³⁺?
Purple —> Dark Green
[5.3.1] How is Mn(OH)₂ formed from Mn²⁺?
Mn²⁺₍ₐᵩ₎ + 2OH⁻₍ₐᵩ₎ —> Mn(OH)₂₍ₛ₎
[5.3.1] What is the colour change in the formation of Mn(OH)₂ from Mn²⁺?
Pale Pink —> Buff
[5.3.1] What happens when you add excess NH₃ to [Cu(H₂O)₆]²⁺?
It will initially form a Cu(OH)₂ precipitate.
Eventually it becomes a ligand substitution reaction, and becomes [Cu(NH₃)₄(H₂O)₂]²⁺.