Weak Acids and Bases
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
equation for a strong acid
dissocates fully
HA→ H+ + A-
equation for a weak acid
dissolves slightly
in dynamic equilibrium with dissocates products
HA ⇌ H+ + A-
what is extent of dissocation
proportion of molecules that dissocate in solution
relationship between bond strenght and extent of dissocation
weaker bonds , dissocate more easily
diffrence between concentration and strenght of acids
strenght of an acid refers to extent to where an acid dissocates into [H+]
conc of acid refers to number of moles of acid in a particular volume of water
what kc for weak acid
Ka acid dissocation contant
what is the equation of acid dissocation constant
Ka = [H+] [A-] / [ HA]
units of weak acids
mol dm-3
what is acid dissoacation constant
quantify and compate strenght of diffrent weak acids
measure proprtion of molecules that dissocate into ions
relationship between Ka nad strenght of acid
increase Ka , increase strenght of acid
to calculate Ka to pH what 2 assumptions
[HA] = [Congugate base (HA)]
[HA] at equilibrium = [HA] initial
how to calculate pH of weak acid
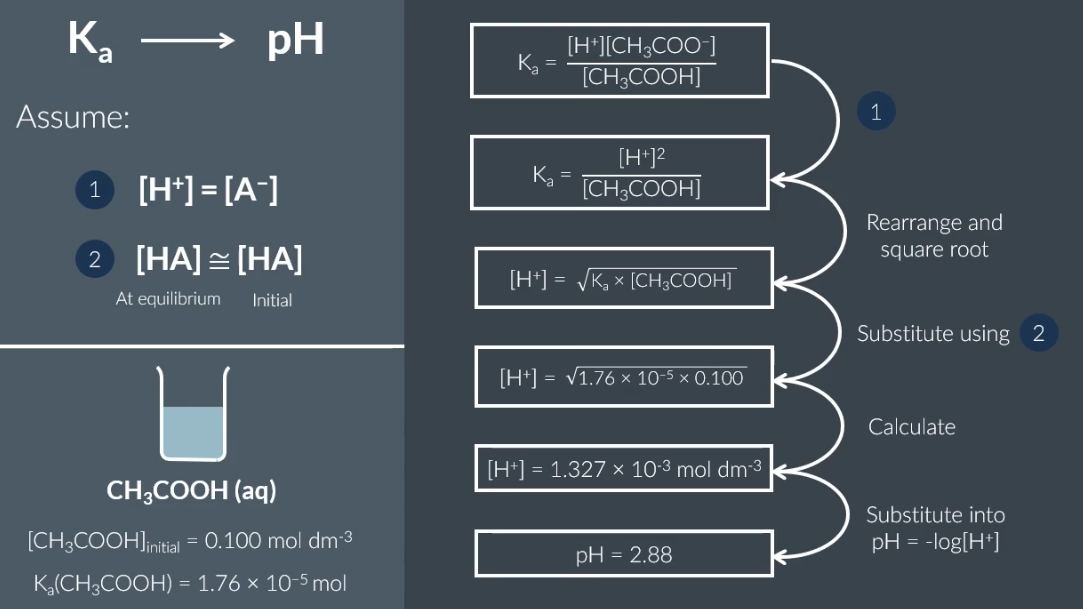
how to calculate concentration of weak acid
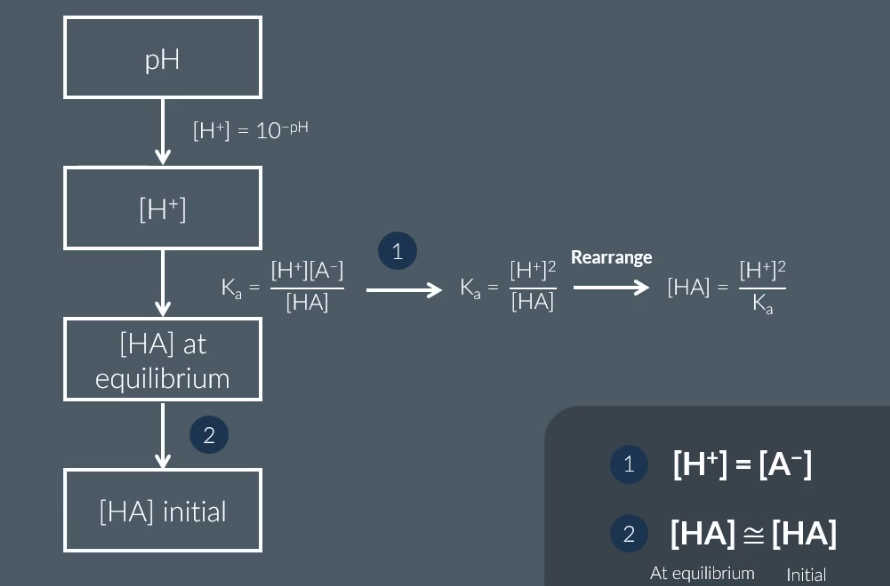
how to calculate pKa
pKA = log (Ka)
relationship between acid strength and pKa
stronger the acid , the lower the pKa
what is a monoprotic acid
an acid that can donate only one proton (hydrogen ion) per molecule in a chemical reaction
what is diprotic acid
an acid that can donate 2 protons per moleule in a chemical reaction
what is a tripotic acid
an acid that can donate 3 protons per molecule in a chemical reaction
what happens to dissocation strength as order of acidic protons dissocate
1st proton dissocates strongly
2nd, 3rd proton dissocate slightly