Organic Chemistry ACS Final Exam
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
64 Terms
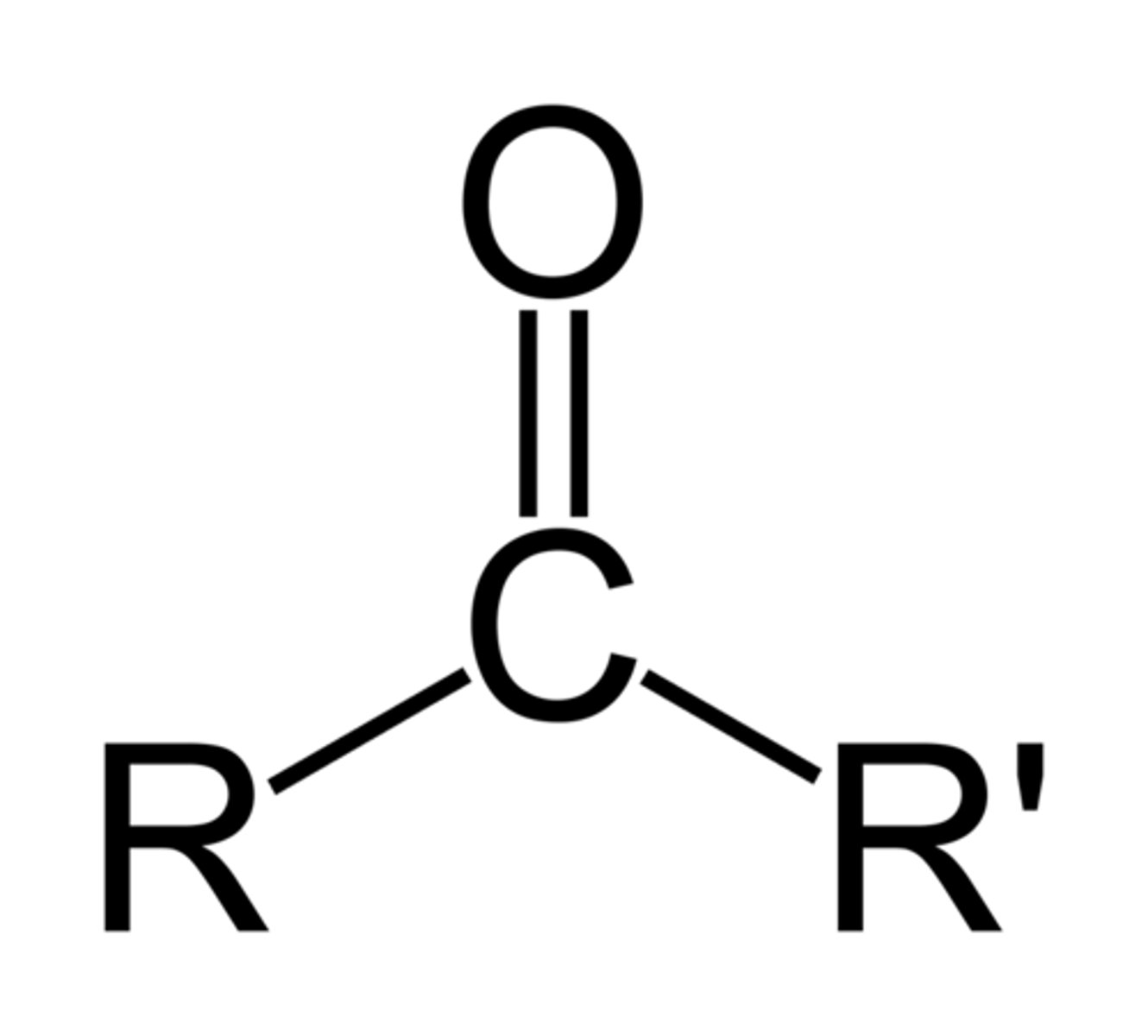
ketone
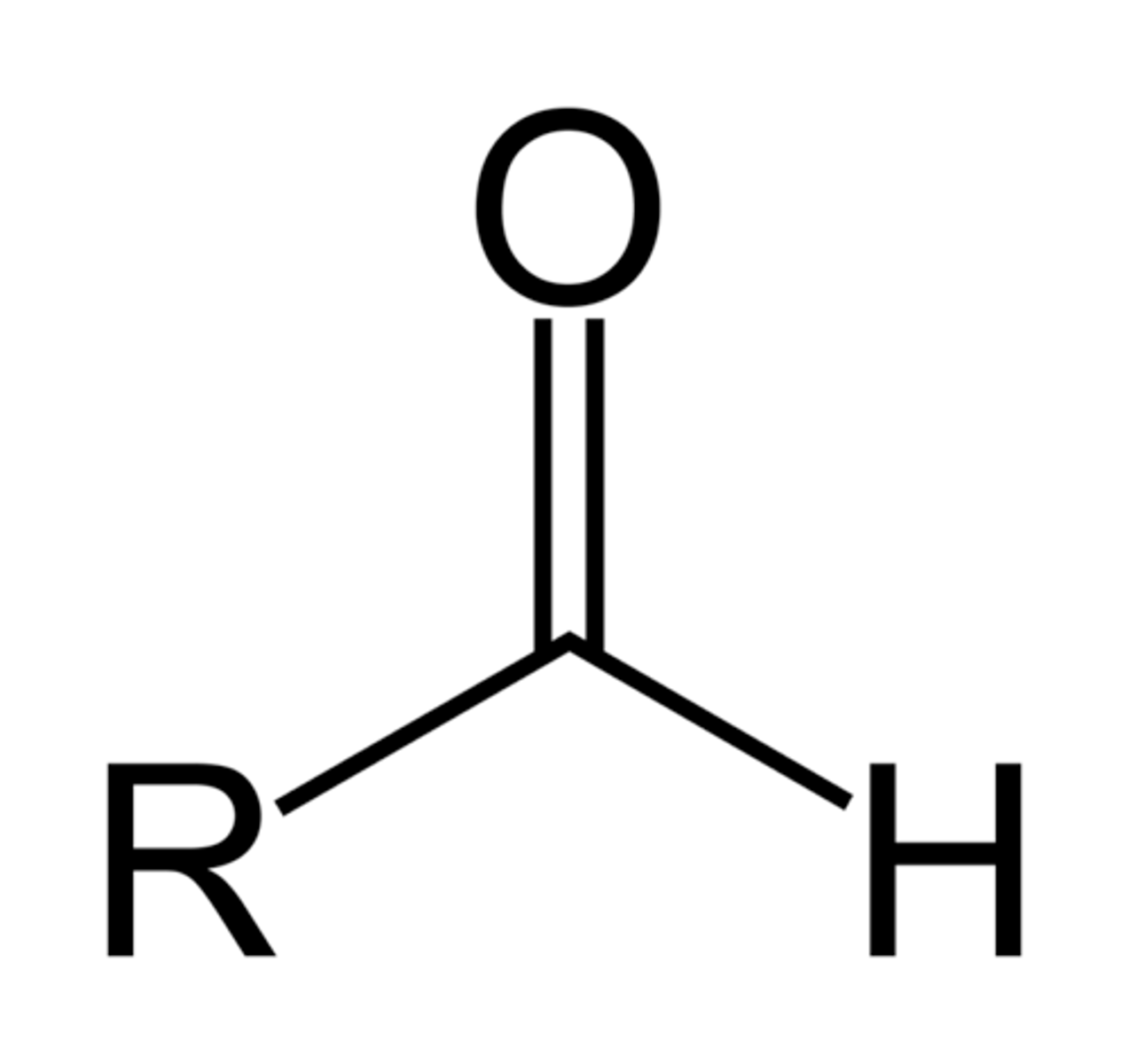
aldehyde
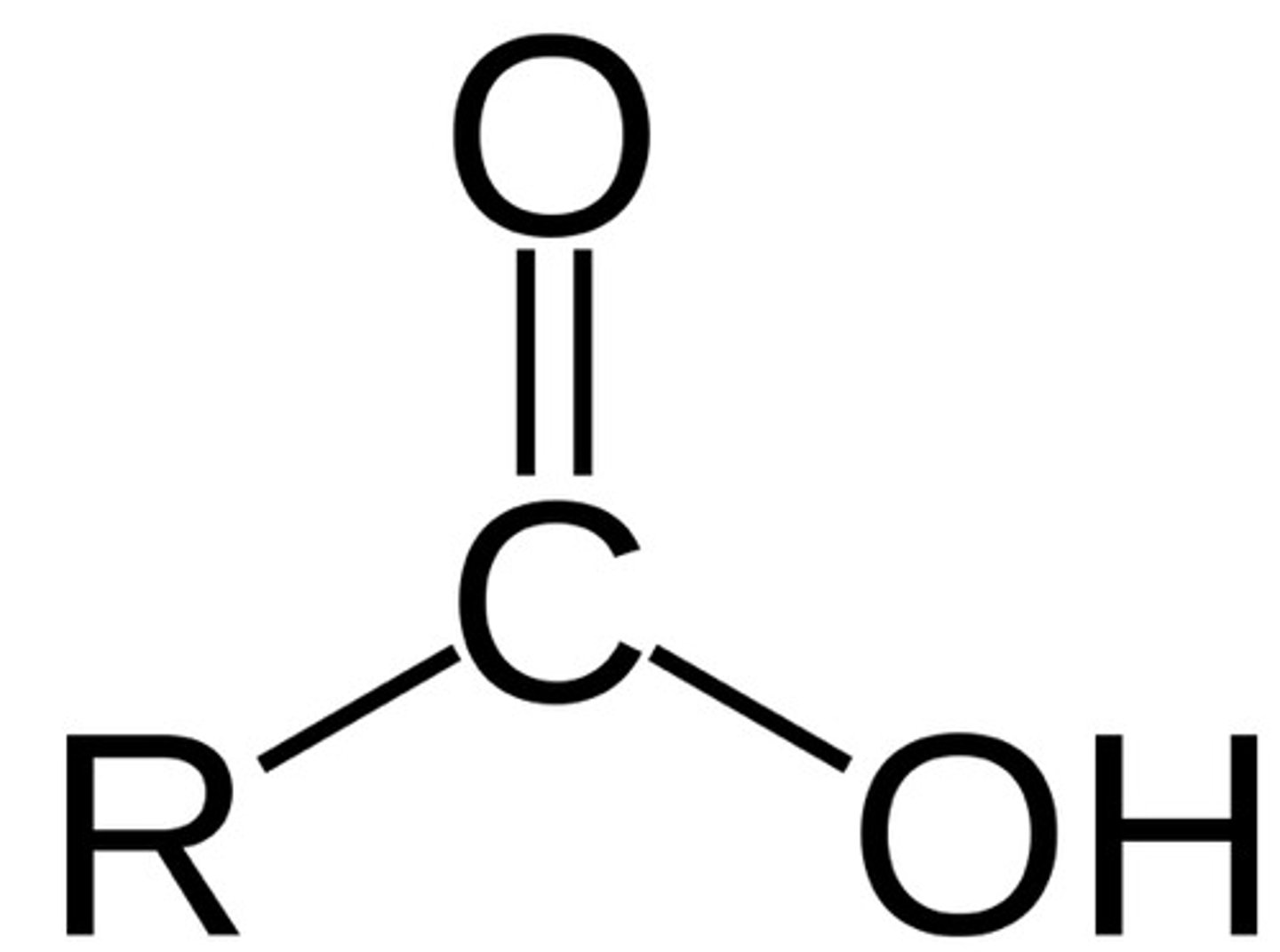
carboxylic acid
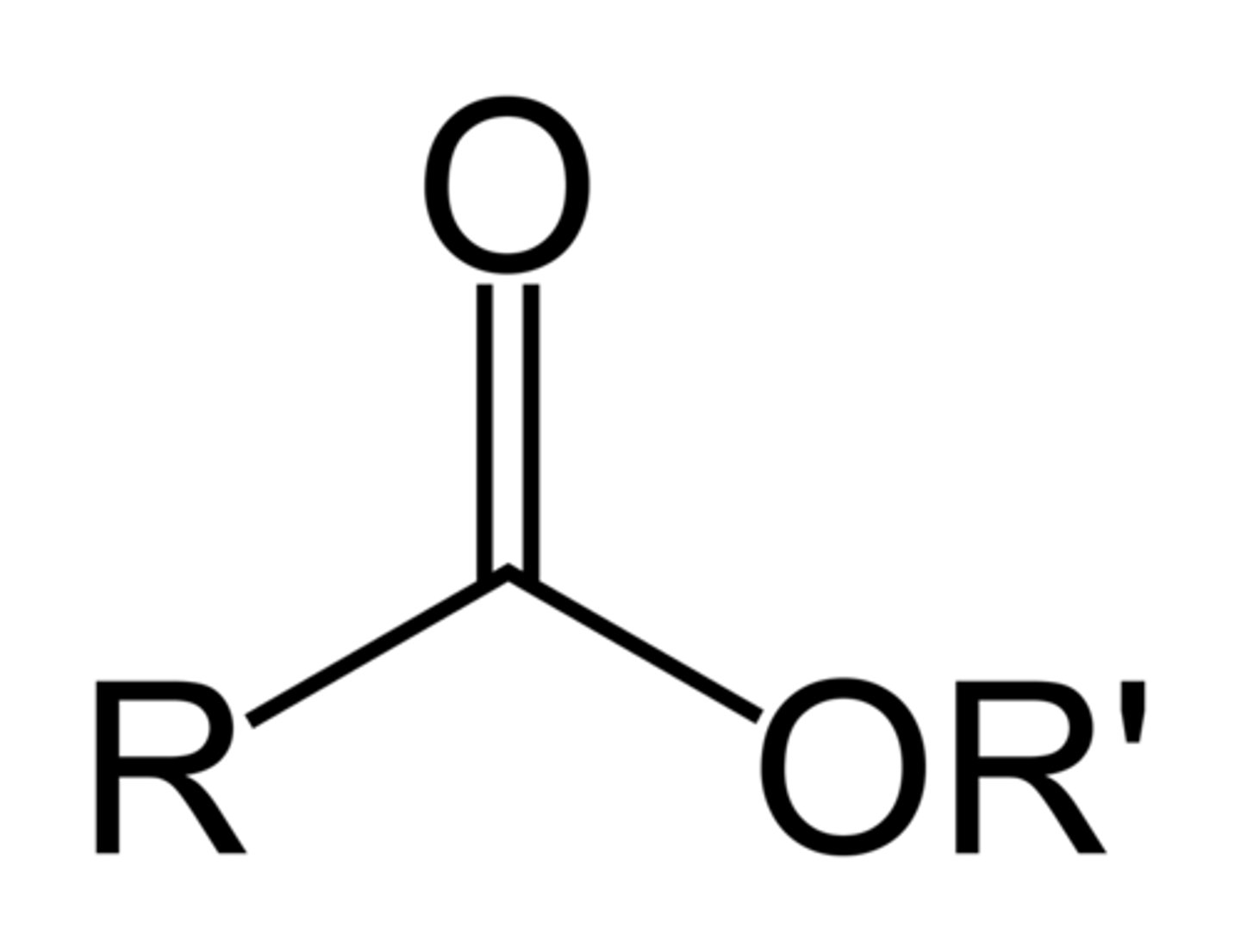
ester
amide
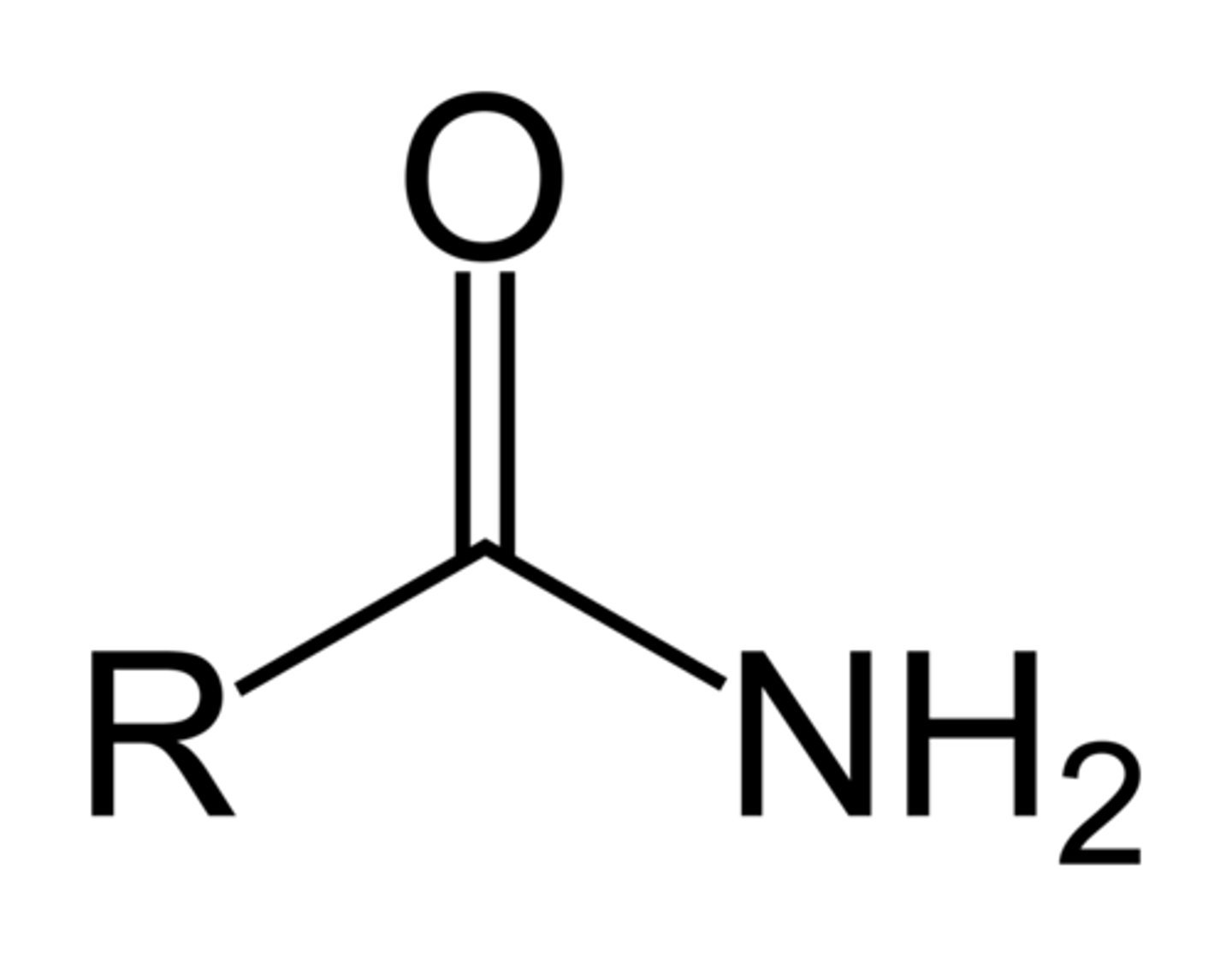
anhydride
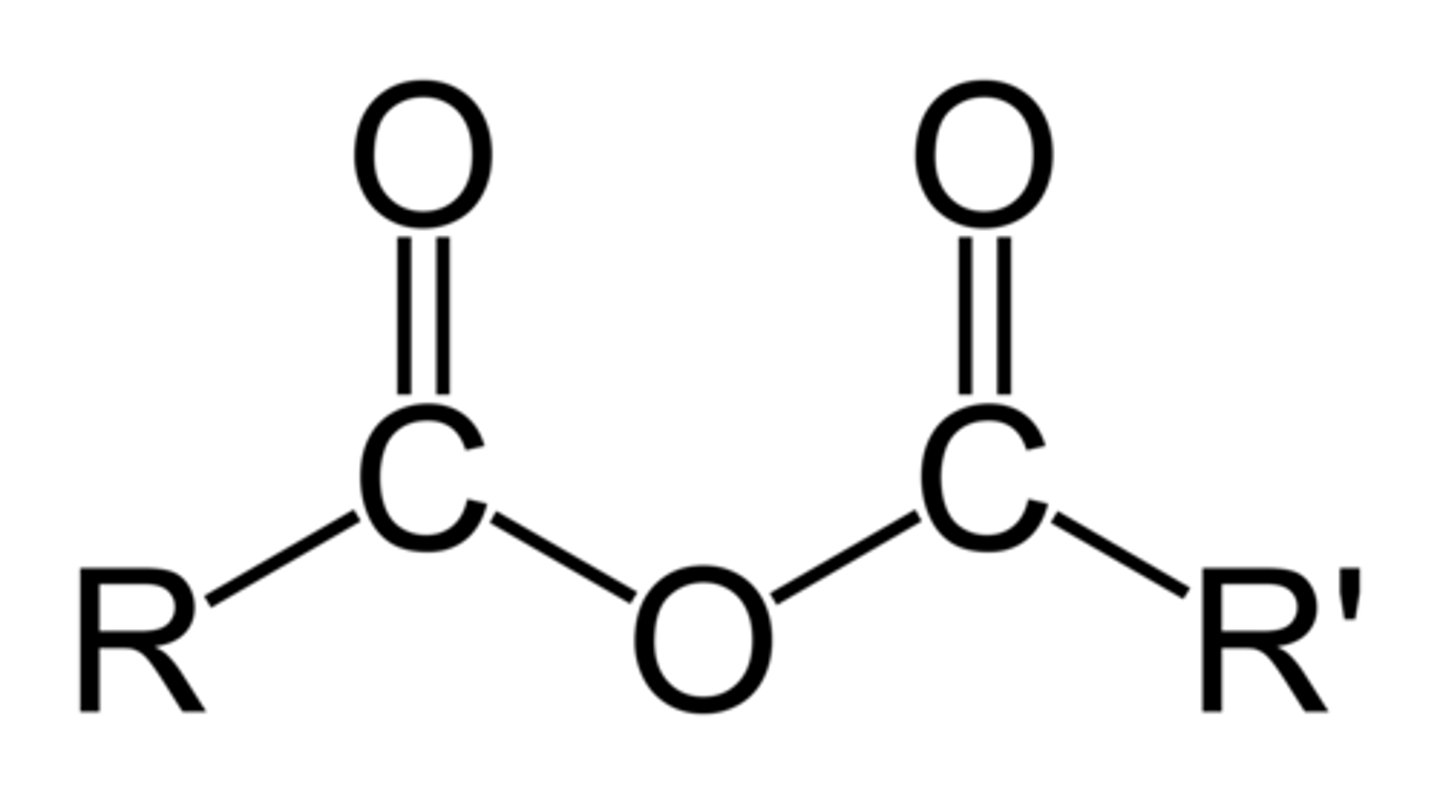
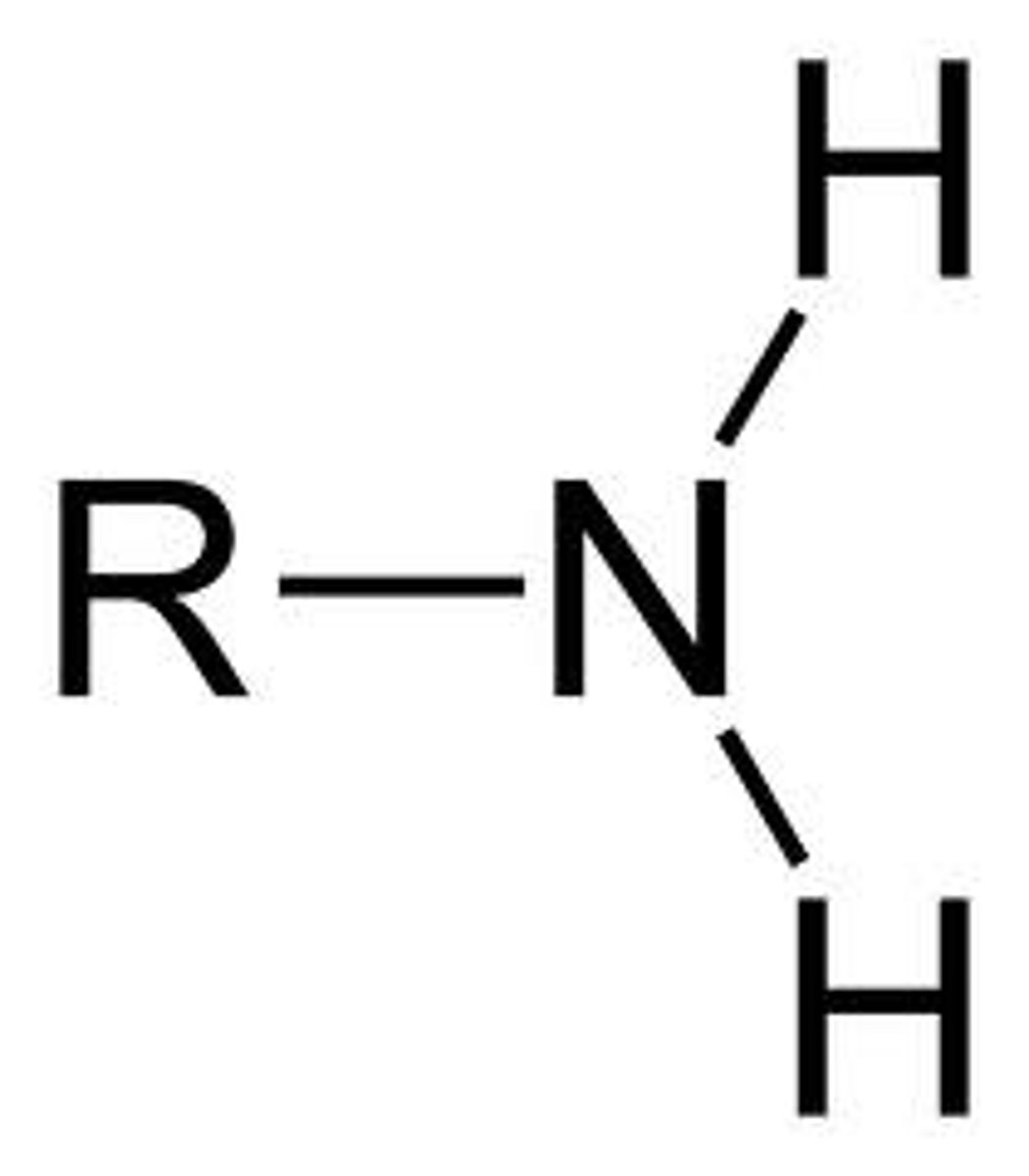
amine
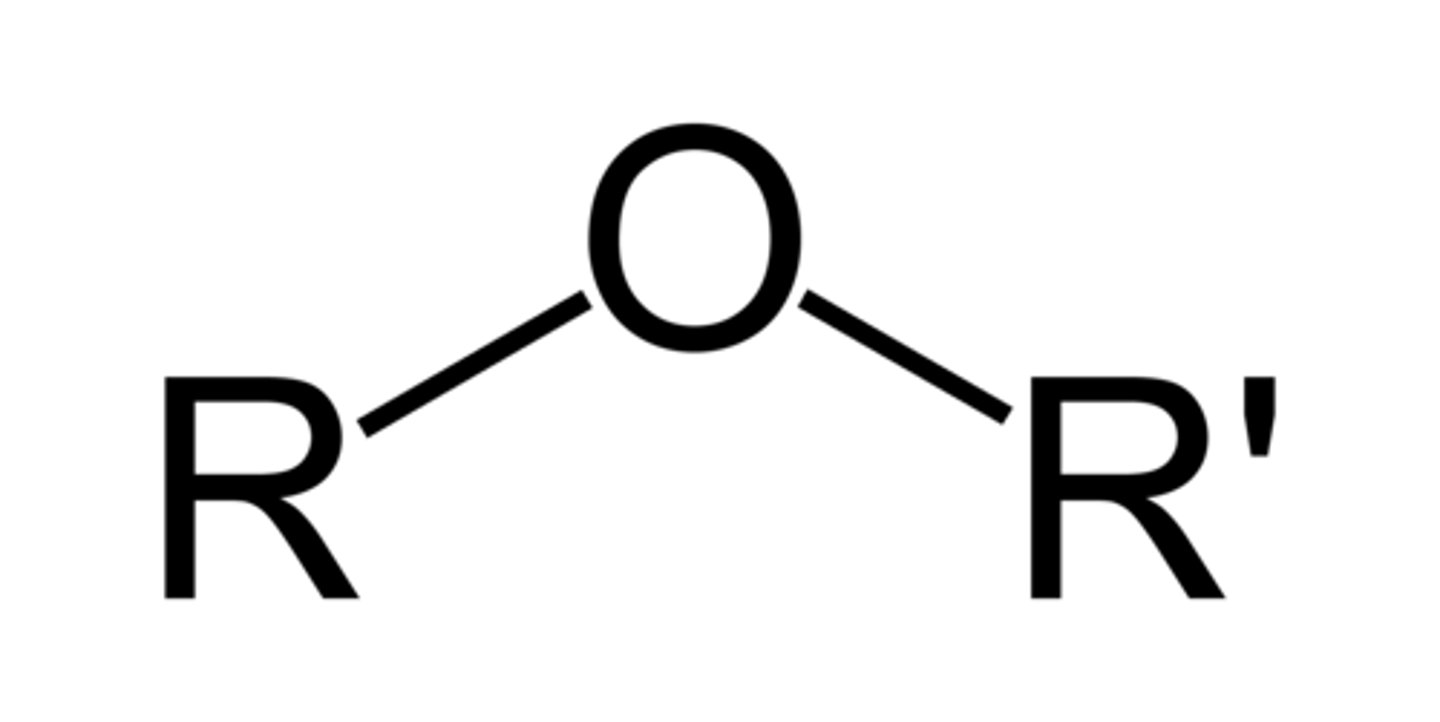
ether
constitutional isomers
same formula, different connectivity
stereoisomers
same formula, different spatial arrangement
enantiomers
non-superimposable mirror images
diastereomers
stereoisomers that are not mirror images of each other
R configuration
clockwise
S configuration
counter clockwise
what functional groups does a peptide bond contain
amide
what functional groups does a lipid contain
esters
what functional groups do amino acids contain?
aromatic rings
meso compound
molecule with multiple stereo centers and it is superimposable on its mirror image
NO2 prefix
nitro
CH=CH2 prefix
vinyl
CH2CH=CH2 prefix
allyl
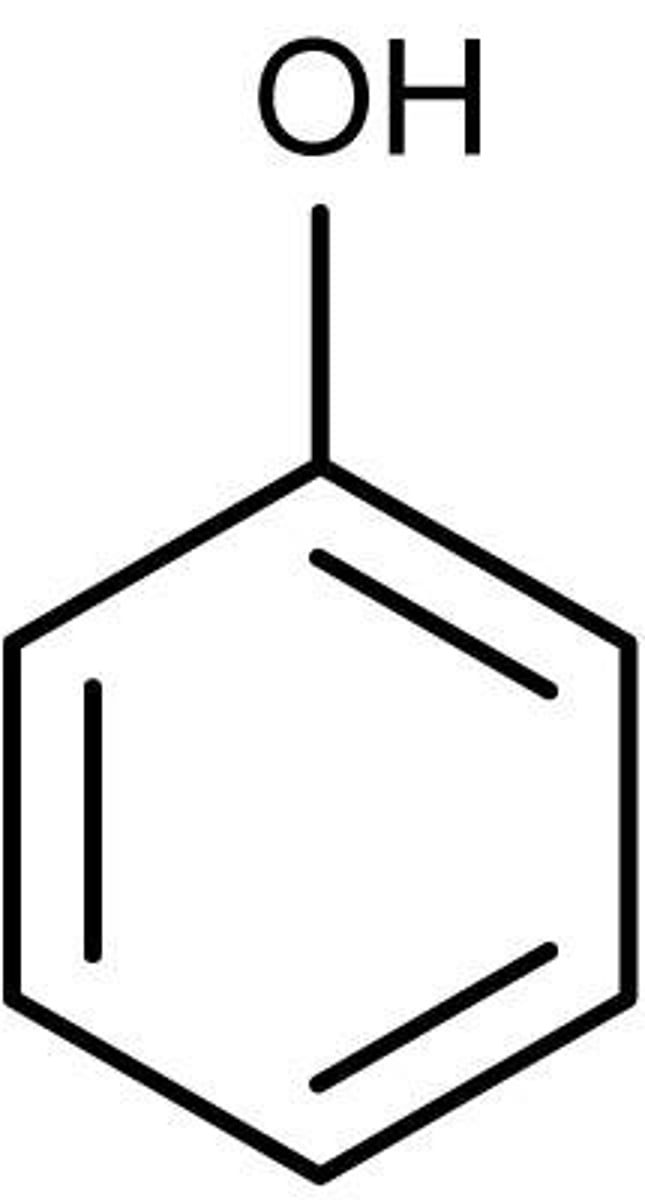
phenol
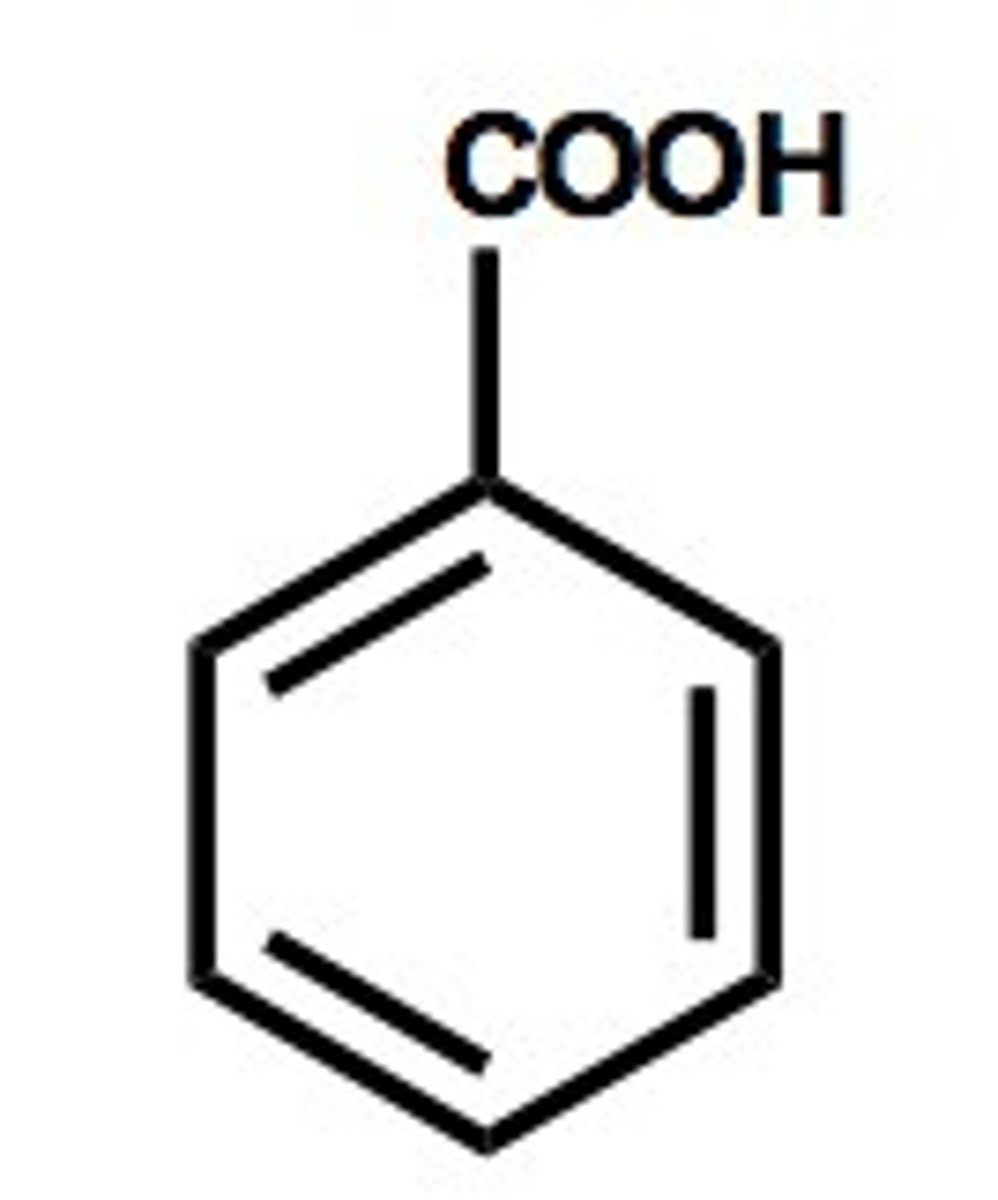
benzoic acid
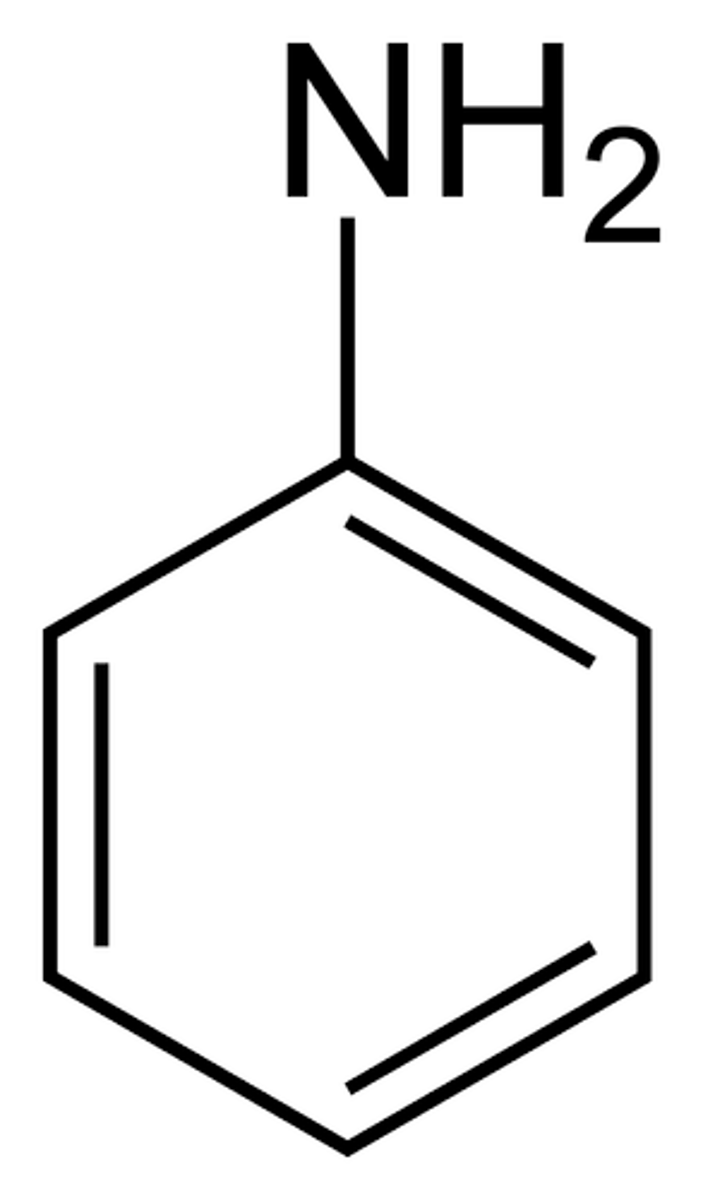
aniline
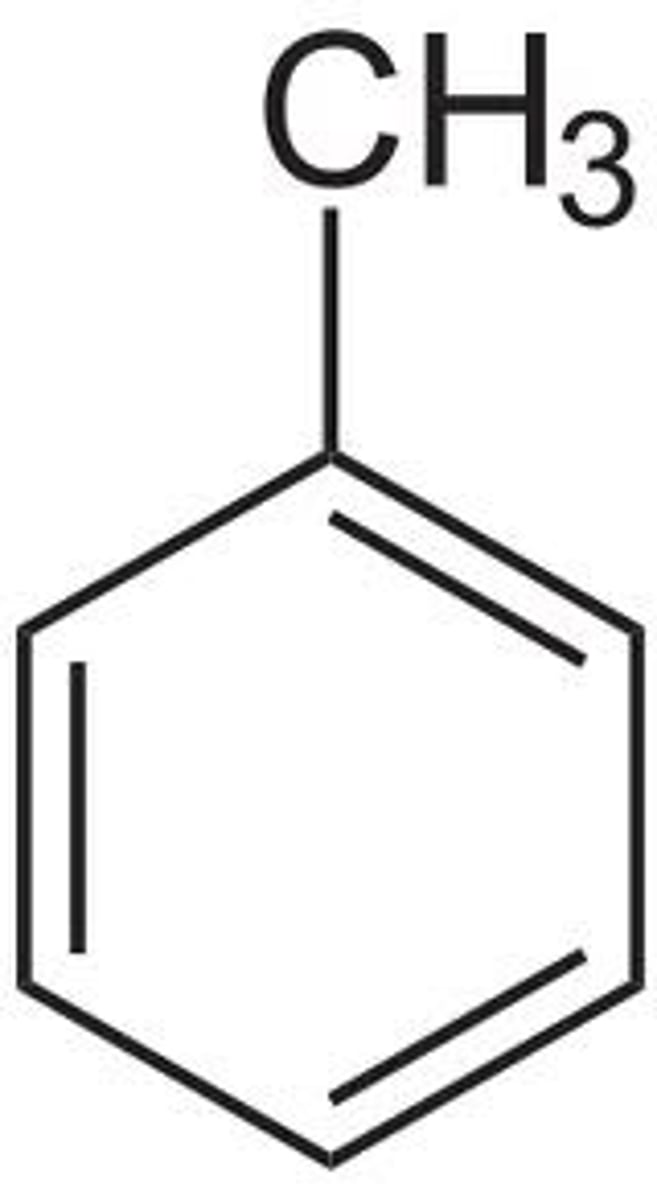
toluene
three types of disubstituted benzenes
ortho-1,2
meta-1,3
para-1,4
only use when you have ONLY 2 substituents
benzene
as you increase heat of combustion, what happens stability?
it decreases
Carbon 13 NMR Resonances
0-80 sp3c
125-150 aromatic
160-180 esters/acids
200-220 aldehydes/ketones
H 1 NMR Resonances
0.9-1.5 sp3 C
2-2.5 allylic H
3-4.5 O-CH3
4.5-6.5 vinylic
7-8.5 aromatic
10 aldehyde
10-12 carboxylic acid
IR Resonances
3400 OH and NH
3100-3000 sp2 C
2100 triple bonds
1700 ketones
1600 C=C, aromatic C-C
1500 aromatic C-C
what is the difference between phenyl and benzyl?
benzyl has CH2 in between the ring and the substituted chain
bond angle for sp2 hybridized?
109.5
what are 2 key things to lewis structures?
1. filled octets
2. electronegative atom has negative charge
if a ring has a charge, then it is ____
aromatic
a strong acid corresponds to?
a weak base
what does a double bond right next to acidic H mean?
it is more acidic
low pKa value means it is...
more acidic
how does hybridization relate to acidity?
the more S character it is, the more acidic
sp>sp2>sp3
a hydrogen next to an electronegative atom will always be...
more acidic
how do you determine if molecules are meso isomers?
needs to be chiral and needs to have a plane of symmetry
how do you find most stable newman projection?
it will have less gauche interactions
what are wedges and dashes in fischer projections?
horizontal-wedge
vertical-dash
if only one stereo center is flipped in a molecule, they are...
diastereomers
do racemic mixtures get plane polarized light?
no, they cancel each other out
do meso compounds get plane polarized light?
no, they cannot bend light
what can triple bonds not do stereoisomerically?
exhibit cis or trans
what can an enantiomer not have?
a plane of symmetry
what happens with a dehydrohalogenation reaction?
H wants to attack and form a double bond to increase stability
a strong acid corresponds to a ________ nucleophile
weak
how do you tell if it is a syn or anti addition?
Syn- two atoms are either both forward/back
Anti-one atom forward and one back
where does hydroboration put the substituent?
on the less substituted side (anti-markovnikov)
what are the most stable types of free radicals?
allylic or benzylic
tertiary>secondary>primary
what are the 3 steps of a free radical reaction?
initiation-goes from 0-1 radicals
propagation- goes from 1 to another 1
termination-goes from 2-0 radicals
what is the energy of propagations like?
very low activation energy
what does non-regioselective mean in terms of energy?
it goes down in energy
what does it mean if an alcohol absorbance peak on the IR spectrum is abnormally wide?
it is a carboxylic acid
what does chromic acid do?
it turns C-H bonds into C-O bonds
if a molecule does not have C-H bonds, it will be unreactive with chromic acid
E2 reactions favor ______ alkyl halides
tertiary
what is the trick for predicting major products of E2 reactions?
if the base is sterically hindered, then the major product will be the LESS substituted alkene
if the base is NOT sterically hindered, then the major product is the more substituted alkene
what is the reagent BH3 * THF used for?
hydroboration-oxidation
how could you tell if a pair were resonance structures?
atoms cannot be moved, only pi bonds and lone pairs
which is more acidic: OH or NH?
OH
which hybridization is the most acidic?
sp>sp2>sp3