Chap 19B - Solubility
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
What is selective precipitation
A solution containing different metal ions can be separated using selective precipitation
Process that involves adding a reagent that has a higher tendency to precipitate with 1 of the metal ions, while leaving the other metal ion in the solution
If a ppt has already formed:
Adding more solid will not increase ion concentration
Equilibrium concentration of ion = highest ion concentration = saturation point of solution

Separate mixture containing equal concentrations of Ca2+ and Mg2+
Since Mg(OH)2 and Ca(OH)2 have the same units for Ksp, and from the smaller Ksp value of Mg(OH)2, Mg(OH)2 is less soluble than Ca(OH)2.
Mg2+(aq) + 2OH−(aq) ⇌ Mg(OH)2 (s)
On adding aqueous NaOH to the mixture, [OH−] increases such that the ionic product of Mg(OH)2 exceeds its Ksp value -> precipitation of Mg(OH)2 occurs.
Since the Ksp of Ca(OH)2 is much higher, for the same concentration of [OH–], the ionic product of Ca(OH)2 does not exceed its Ksp value -> precipitation of Ca(OH)2 does not occur
Since Mg2+ precipitates out as Mg(OH)2 while Ca2+ remains in aqueous solution, filtration can be effected to separate the two
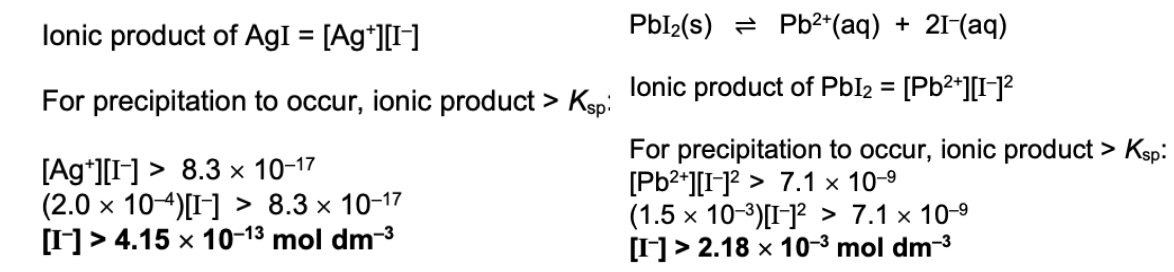
State conclusion for this
Since minimum [I–] to cause precipitation of AgI is smaller than that of PbI2, AgI will precipitate out first
State factors of solubility
Common ion effect
Complex ion formation
Effect of pH
Describe common ion effect explanation (Eg. Addition of NaCl(s) to a saturated solution of AgCl(s))
Presence of a common ion reduces the solubility of a salt as it will favour precipitation
Addition of NaCl(s) to a saturated solution of AgCl(s):
NaCI -> Na+ + CI-
The addition of NaCl introduces the common ion Cl–, increasing [Cl–] in equilibrium (1).
This causes [Ag+][Cl–] > Ksp, causing AgCl to precipitate
POE (1) will shift left to counteract the increase in [Cl–], reducing the solubility of the salt
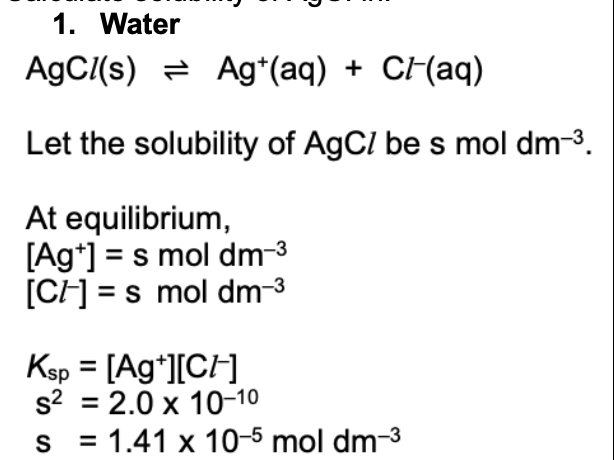
Calculate solubility of AgCI in water
Calculate solubility of AgCI in 0.10 mol dm–3 AgNO3 solution
AgCl(s) ⇌ Ag+(aq) + Cl–(aq)
AgNO3(aq) → Ag+(aq) + NO3–(aq)

Describe complex ion
A complex ion = metal ion at its centre surrounded by ligands
Ligands: Lewis base (has lone pair of electrons to form dative bond with metal ion)
Metal ion: Lewis acid (Has energetically accessible empty orbital to overlap with the orbital containing the lone pair of electrons of the ligand)
Describe complex ion formation when NH3 added to AgCi
The addition of NH3(aq) removes Ag+(aq) due to the formation of the [Ag(NH3)2]+ complex ion, resulting in a decrease in [Ag+].
POE (1) will shift right to counteract the decrease in [Ag+].
When the ionic product, [Ag+][Cl–] is smaller than its Ksp, the AgCl precipitate dissolves, thus increasing the solubility of AgCl