Ochem Flashcards
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
What are the types of intramolecular forces (in order of strength)?
Ionic interactions, hydrogen bonding, induced dipole induced dipole and dipole-dipole
What is the only bending vibration on the IR?
N-H
What is a polymorph?
It is the same molecules however with different packing, properties and intramolecular interactions
How many Cs are water soluble per polar group?
4-5
What is mixed melting point used for?
an ID method
not purity
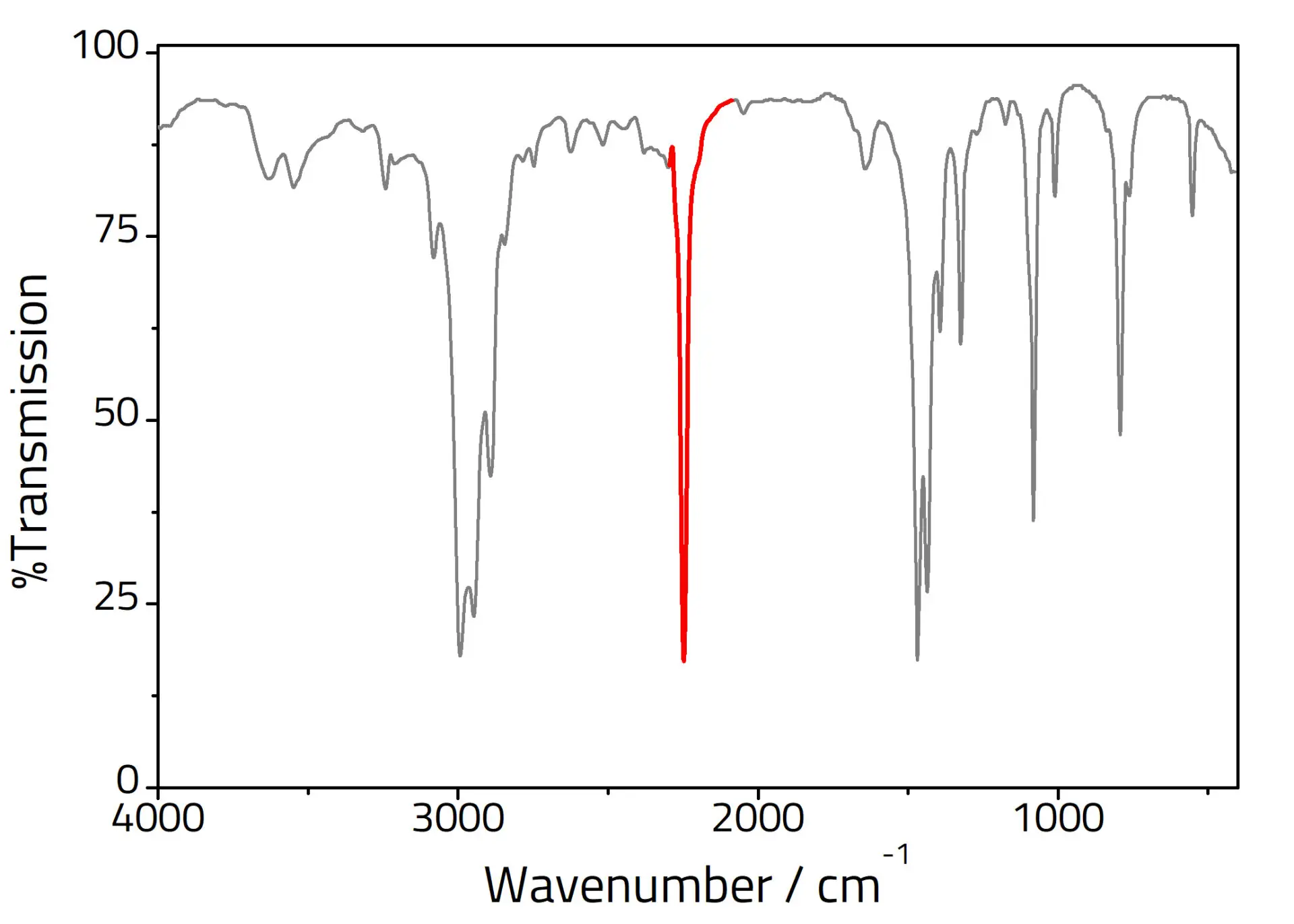
What strong peak is usually present around 3000 cm?
C-H
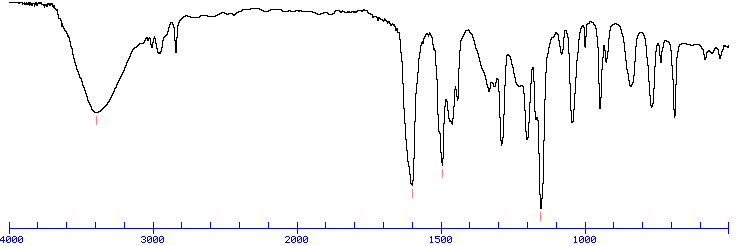
What is a broad stretch usually around 3000 cm?
O-H
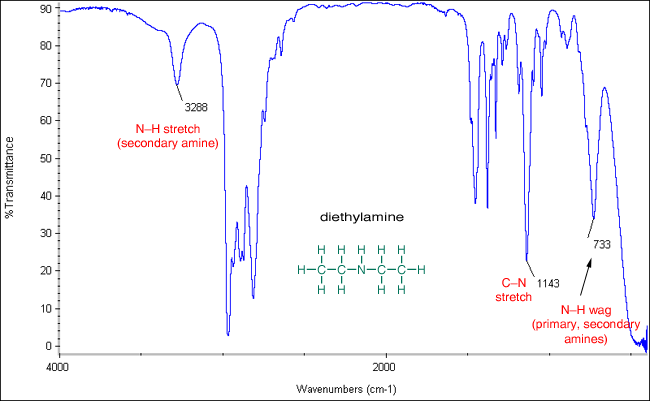
What is one fang around 3200?
nh
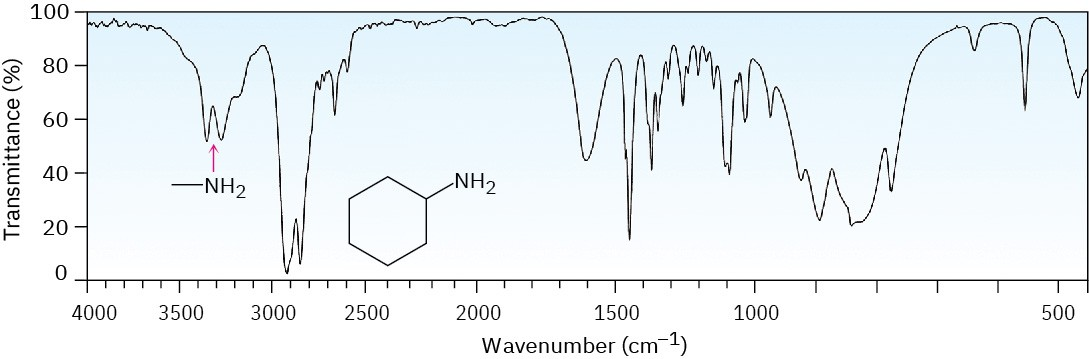
What has the double peaks around 3200?
Nh2
What is he hair beard around 3k on ir?
COO
What should be checked in region 3 of the IR?
carbonyl
What is a polymorph?
same molecules different packing, different properties and different intramolecular interactions
What is the ideal solvent for recrystalization?
soluble hot, insoluble cold, volatile and non reactive
What are some problems with recrystalization?
if there is similar solubility it cannot be done
What should happen if the solute does not dissolve when doing an recrystallization?
heat and wait
What should you do if your solute does not crystalize?
cool and wait or scratch sides
What happens when the recovery of the recrystallization is low?
too dilute and tare flasks
What does it mean with the crystals bring an high recovery?
not dry, tare issues,
what is enthalpy?
bonds broken - bonds made
What should Kd be for good seperation?
3/2
What is the equation for KD?
[org]/[aq]
What does nrining a solution do?
pulls water out of the solution by making the solution more polar
What is benzoic acids kd in ethyl acetate?
2
What causes the emulsions in our tea solution?
saponins
How are tannins removed?
they perform basic hydrolysis when in the ch2cl2 or na2co3
What does the melting range of a compound tell us about a compound?
a physical property that identifies a compound and is an indication of purity
What are mixed melting points used for?
to identify compounds by mixing them together
does not indicate purity of any compound
How does IR spectroscopy work?
organic molecules absorb infrared light when the energy corresponds to specific molecular vibrations and rotation, the IR spectrum shows the inverted peals at the frequencies where they are absorbed
Do organic or inorganic molecules have higher melting points?
inorganic molecules
What molecules have greater vapor pressur?
smaller molecules, symetric molecules and molecules with smaller dipole moments
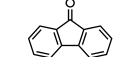
9-fluorenone
How much hot solvent should be added for recrystallization?
the minimum amount of solvent because the concentration of the compound should be high and supersaturated
How can colored soluble impurities be removed?
by adding activated carbon
What can be done if crystals do not form?
adding seed crystal, scratching side of glass, and reheating solution to remove excess solvent
What is liquid-liquid extraction?
separation method based on differential solubility of components in a mixture between two immiscible solvents
What is the insoluble compound present in the teas solution?
cellulose
What are the water soluble compound present in the teas solution?
saponins, tannins and teas protein and pigments
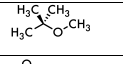
MTBE
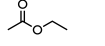
ethyl acetate
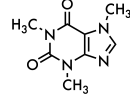
caffeine
benzoic acid
How are acidic organic compounds seperated?
can be turned to water soluble anions using inorganic bases
How are basic organic compounds separated?
can be turned to water soluble cations by treatment with inorganic acids