Physio 3: Electrophysiology
1/97
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
98 Terms
What is diffusion?
initial driving force for the movement of molecules
How do molecules move in diffusion
high to a low concentration (down the gradient)
What types of equilibrium (or disequilibrium) exist in body cells?
Cells of the body are:
In chemical disequilibrium
In osmotic equilibrium
In electrical disequilibrium
Cells in chemical disequilibrium
More Na+ and Cl- outside, more K+ and negatively charged proteins inside.
Cells in osmotic equilibrium
water moves freely across the membranes until osmotic pressure is equalized (290 mosm inside and outside)
Cells in electrical disequilibrium
few extra negative ions inside cells and their matching positive ions are outside
Electrochemical equilibrium
electrical gradient exactly offsets the concentration gradient.
How is electrochemical equilibrium calculated
Nernst equation
Nernst equation
determines the equilibrium (reversal) potential for a specific ion based on its charge and concentration difference across the membrane
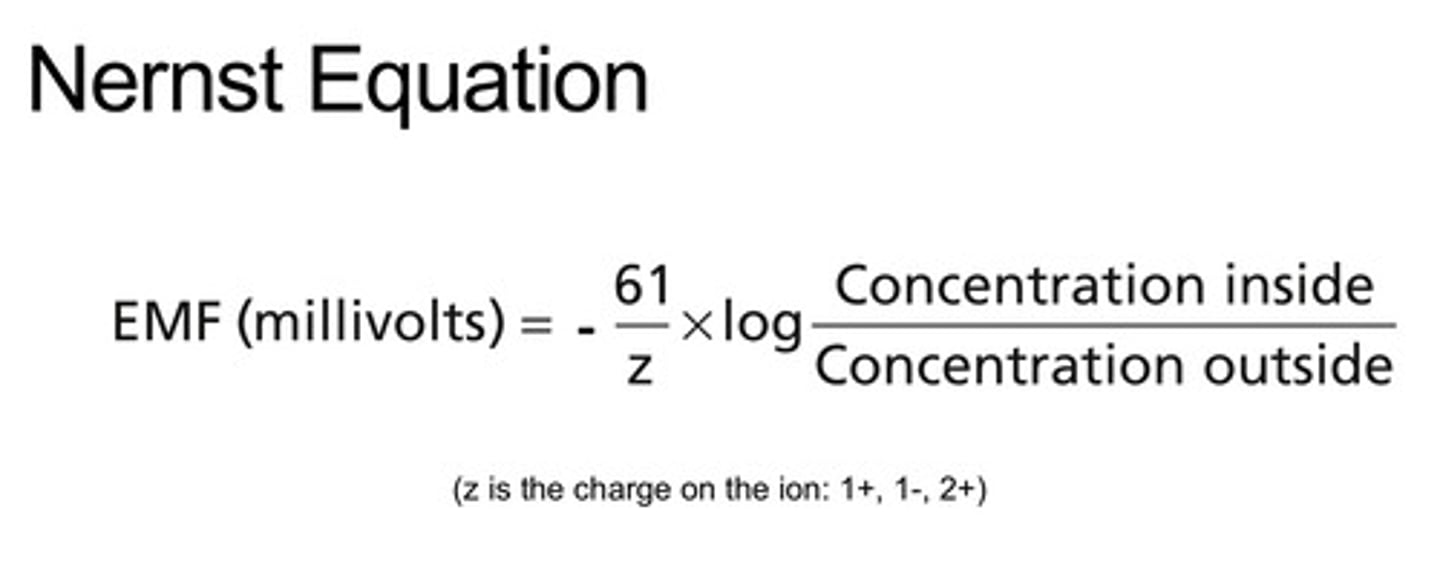
Nernst potential (reversal)
know the numbers
What is membrane potential
Electrical potential difference across a cell membrane. Caused by uneven distribution of ions across a cell membrane.
What ions does membrane potential determine the potential difference for?
ALL ions across the membrane
What factors determine membrane potential?
- concentration of ions in ECF and ICF
- polarity of each ion (+/-)
- permeability of the membrane to the ions
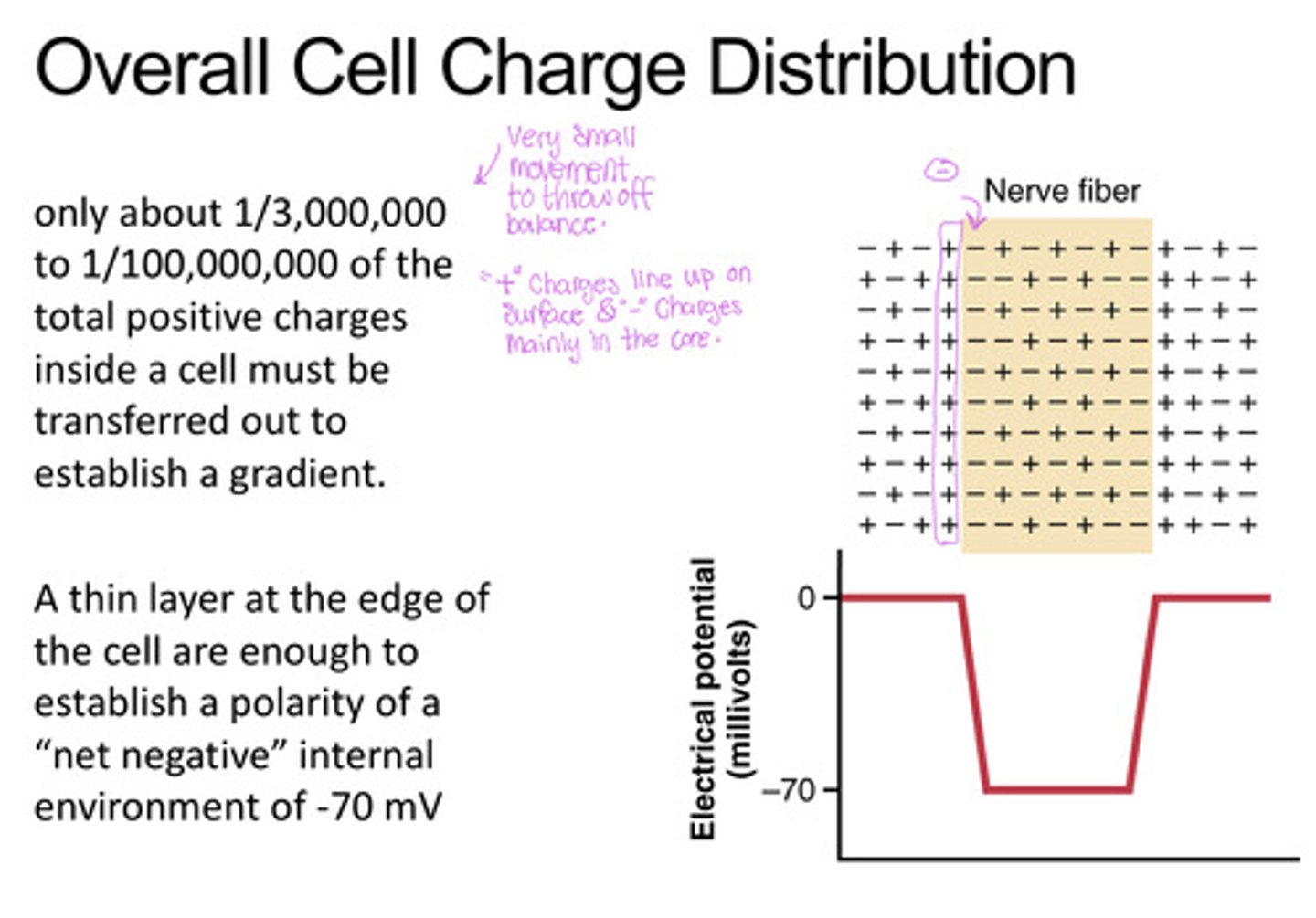
Overall cell charge distribution
+ charges line up on the surface establishing a polarity of a "net negative" internal environment
Goldman-Hodgkin-Katz Equation
predicts membrane potential that results from the contribution of all ions that can cross the membrane (similar to nernst but w/ permeability factored in)
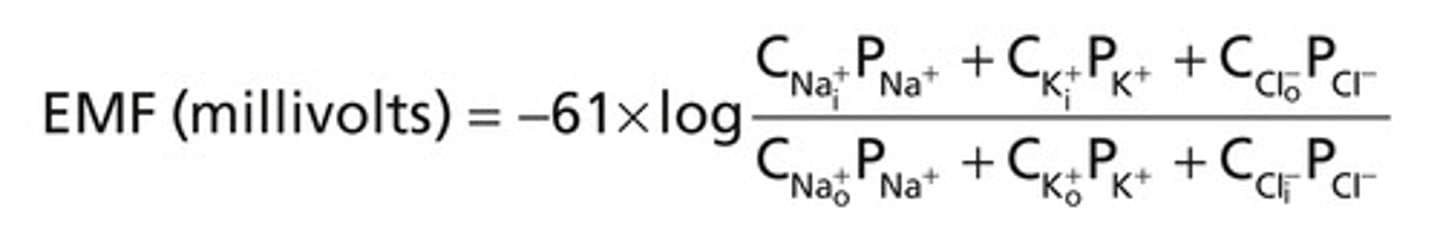
If the permeability of ion = 0, how much do they contribute to the membrane potential
they do not contribute
Permeability = 1, how much does it contribute to the membrane potential
100% permeable
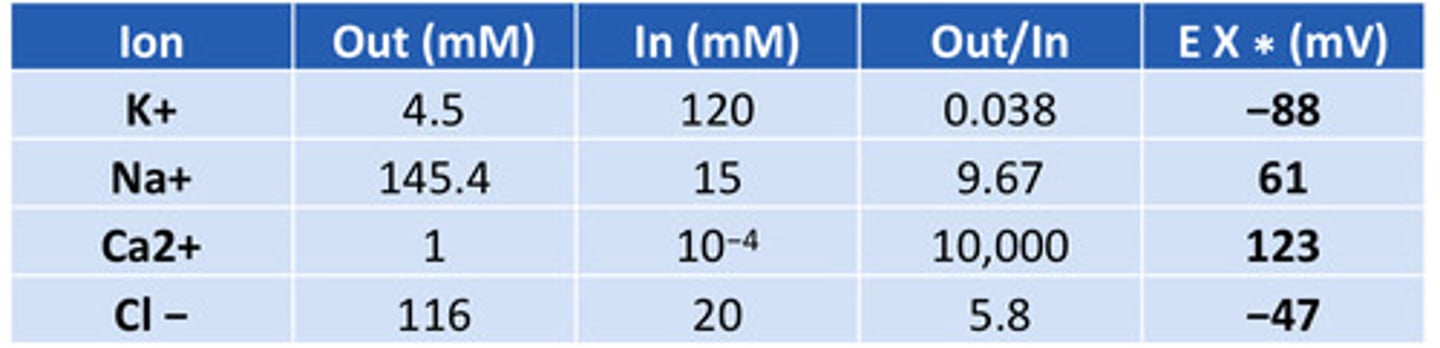
Resting membrane potential table
know the numbers
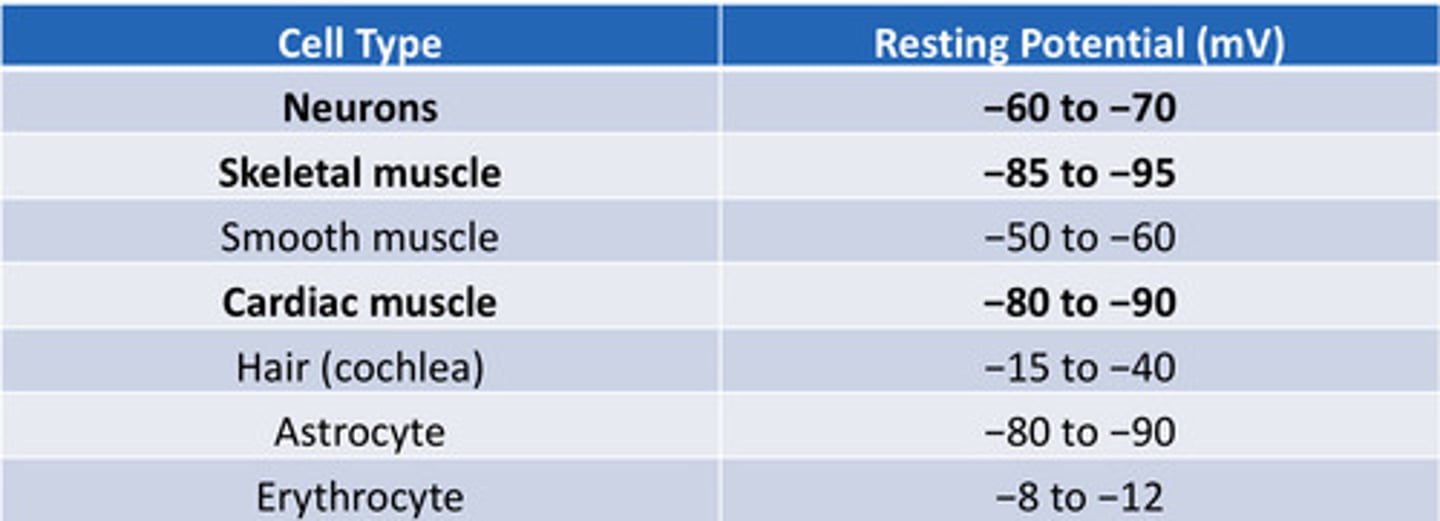
Resting membrane potential of neurons
-60 to -70 mV
Resting potential of skeletal muscle cells
-85 to -95 mV
Resting membrane potential of cardiac muscle cell
-80 to -90 mV
Reversal potential of potassium
- 88 (~ -90mV)
Reversal potential of sodium
+60 mV
Reversal potential of Ca2+
123 mV
Reversal potential of Cl-
-47 mV
What factors contribute to the resting membrane potential
- Na+/K+-ATPase establishing Na and K gradient
- K+ leak channels letting K+ exit, leaving behind (-) charge
- Minor contribution for Na+ influx and the Na+/K+ pump, which adds about -4 mV
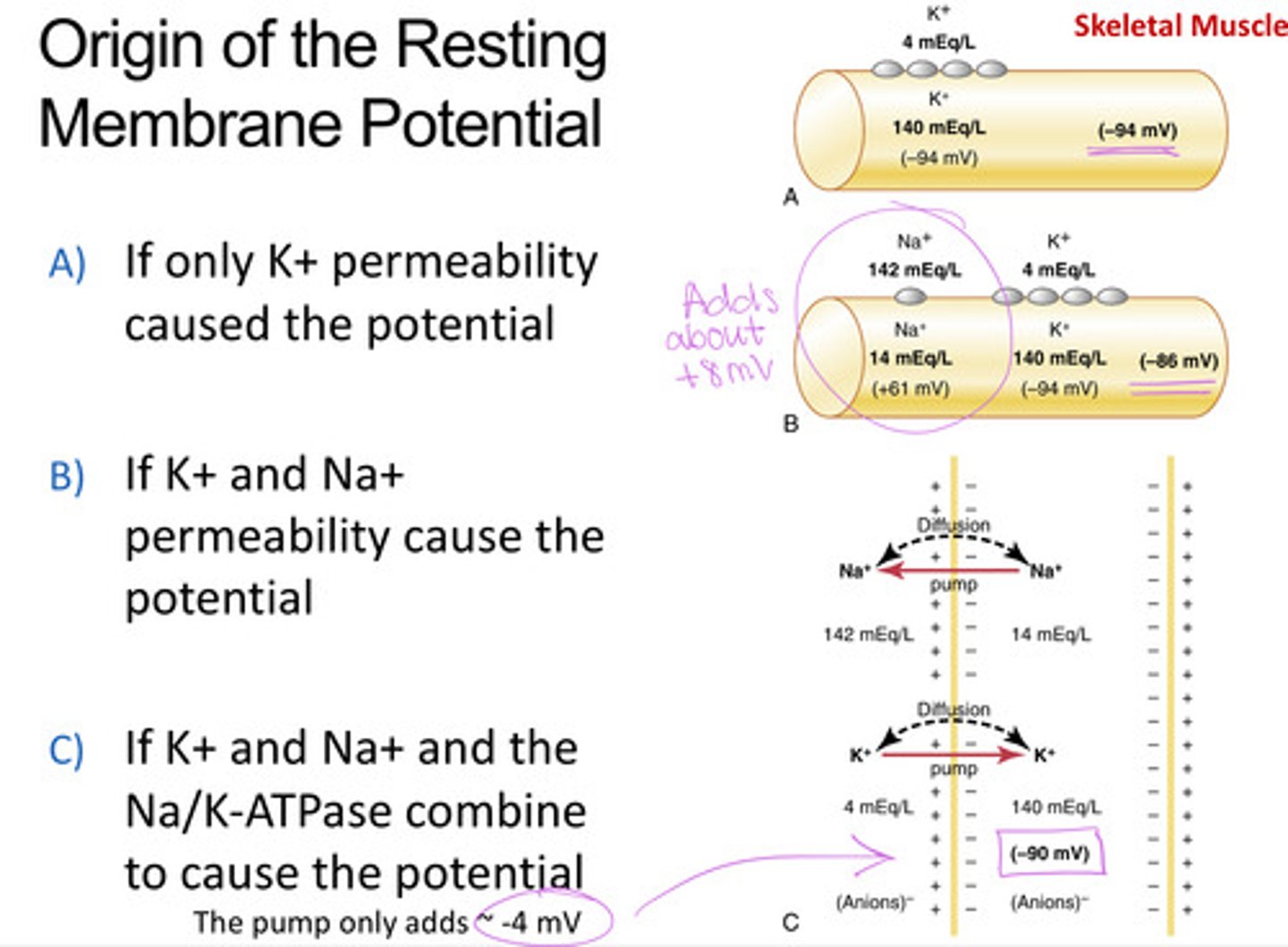
Na concentration inside/outside the cell
14/142 = 0.1 (factor of 10)
K concentration inside/outside the cell
140/4 = 35 (3x10 driving force stronger inside)
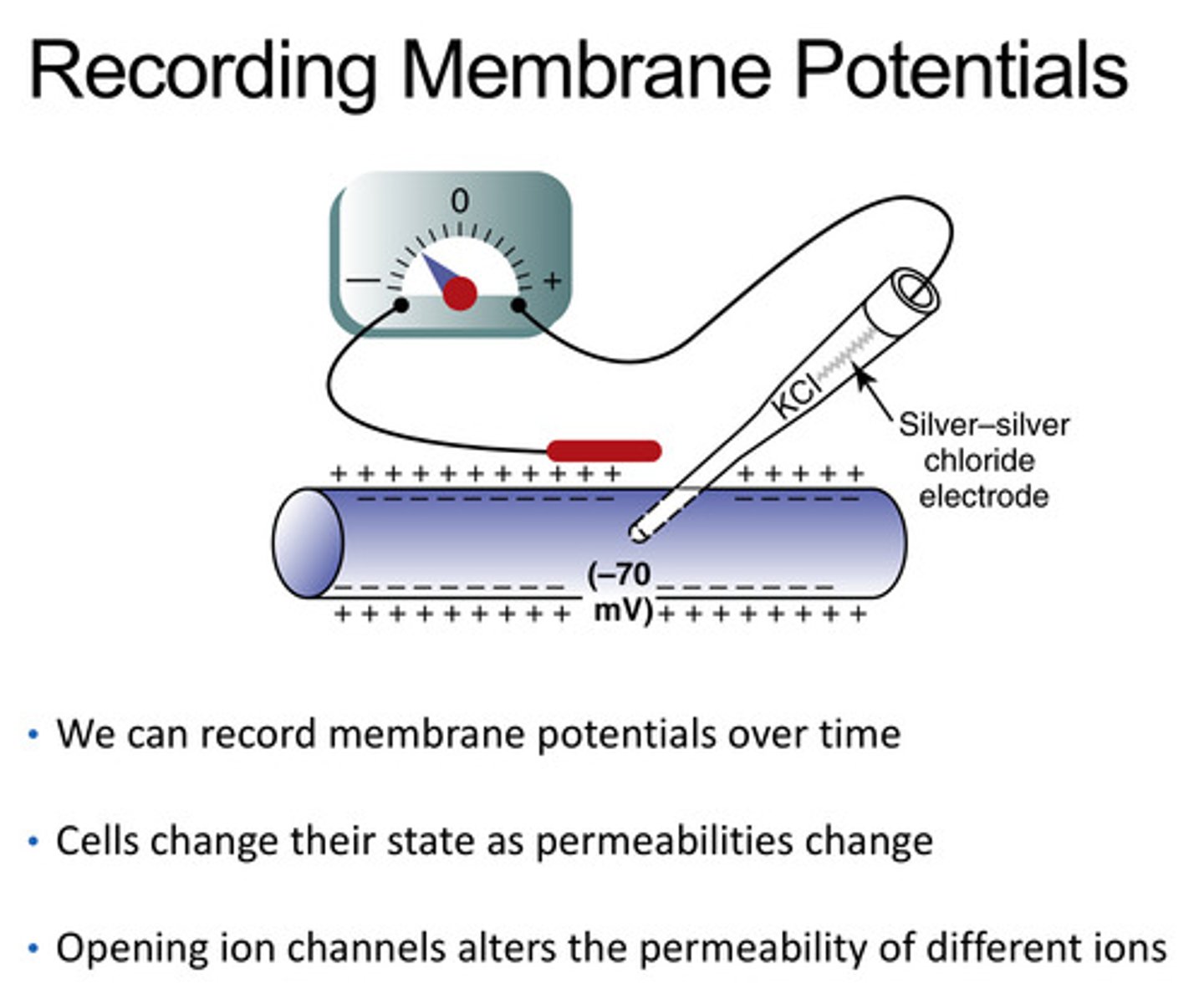
How can we record membrane potentials
with a silver-silver chloride electrode
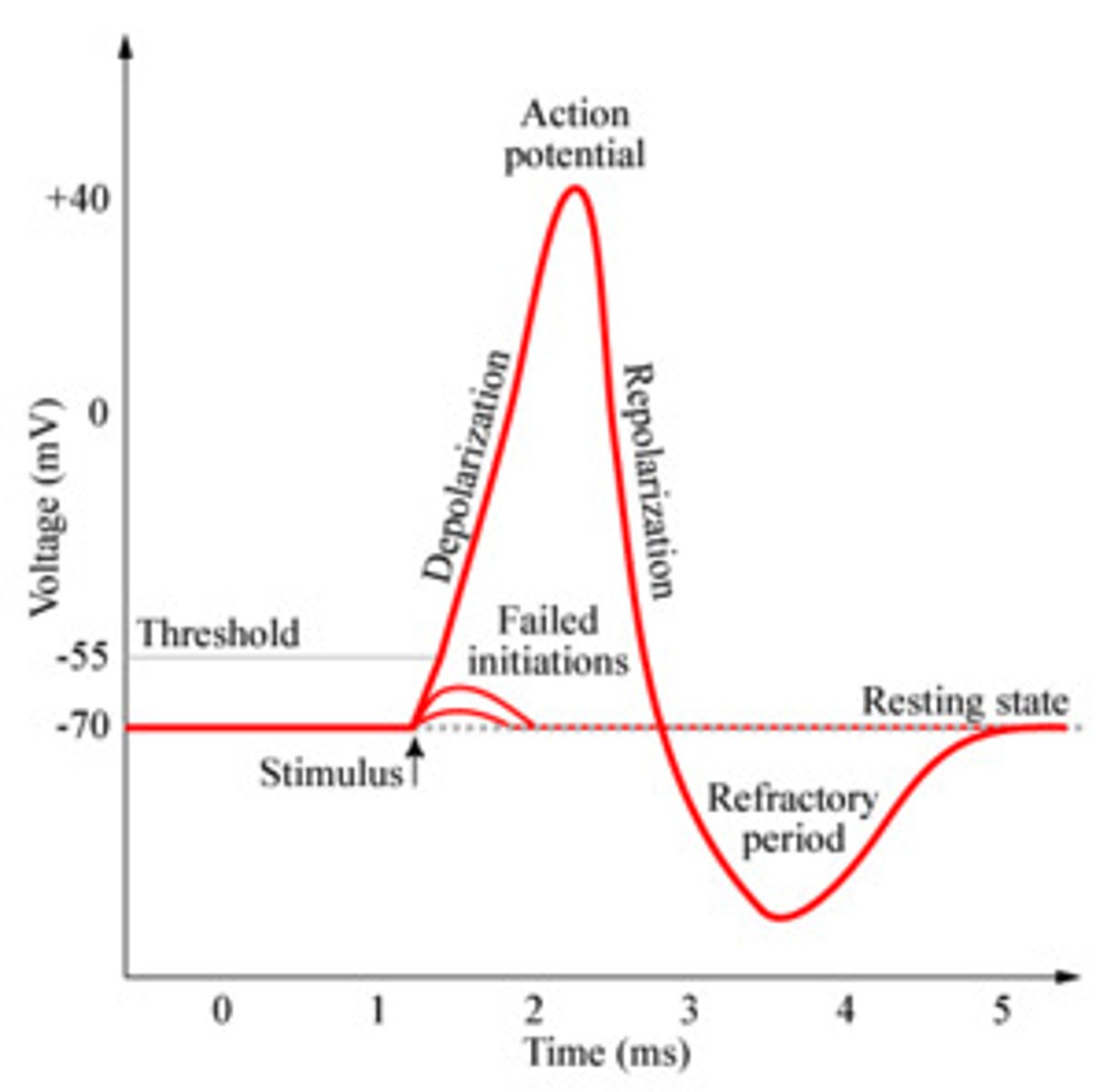
4 main states of an action potential
- resting phase
- depolarization
- repolarization
- hyperpolarization (after potential)
resting potential of a neuron is at
-70mV
threshold potential
The minimum membrane potential that must be reached in order for an action potential to be generated = -55 mV
Role of Na+ in neuronal action potential
drives rapid depolarization (in the positive direction)
Depolarization occurs due to the opening of which channel
Na+ voltage gated channels
Repolarization occurs due to the opening of
K+ voltage channels open back to rest
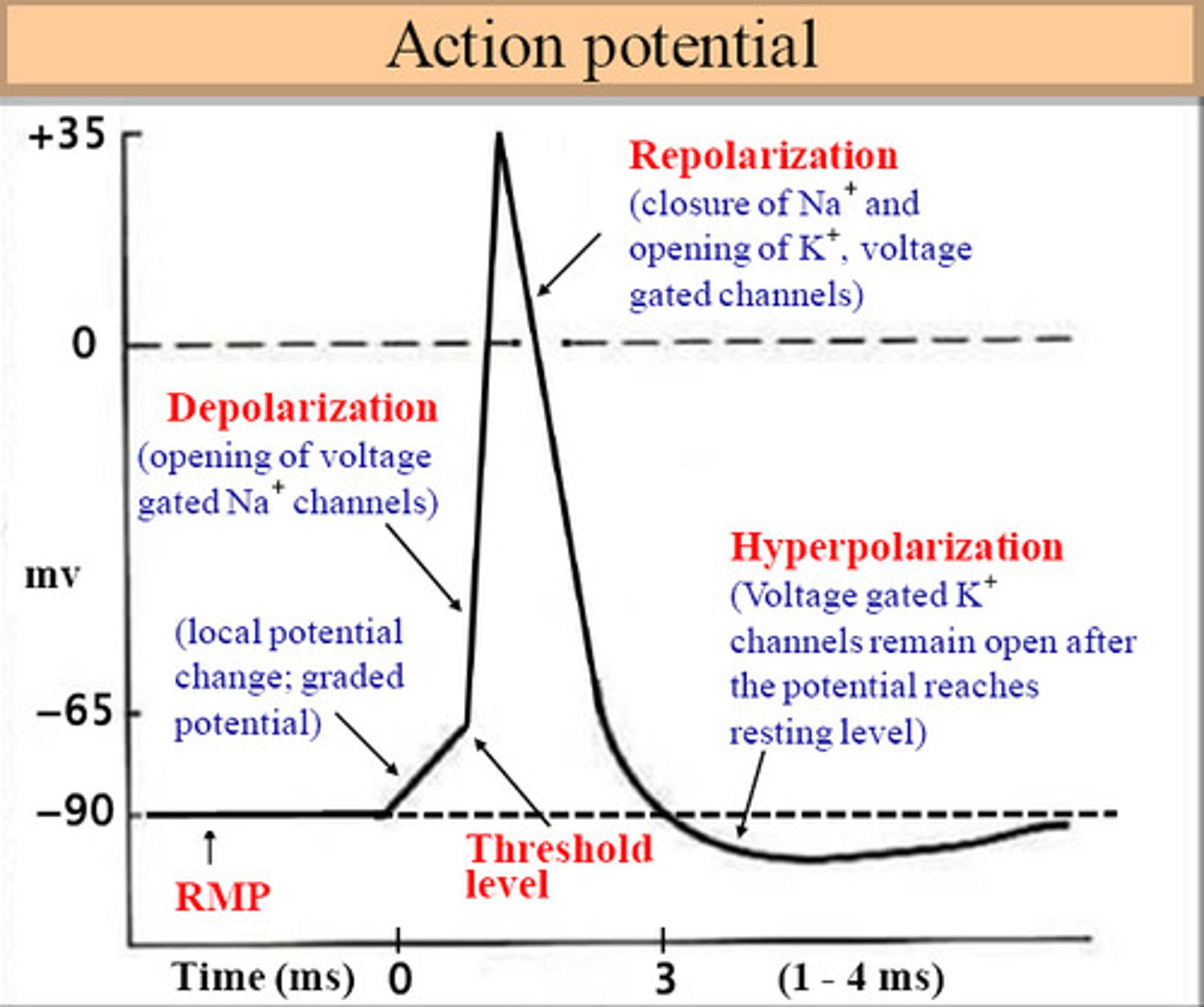
hyperpolarization phase
membrane potential temporarily becomes more negative than resting membrane potential
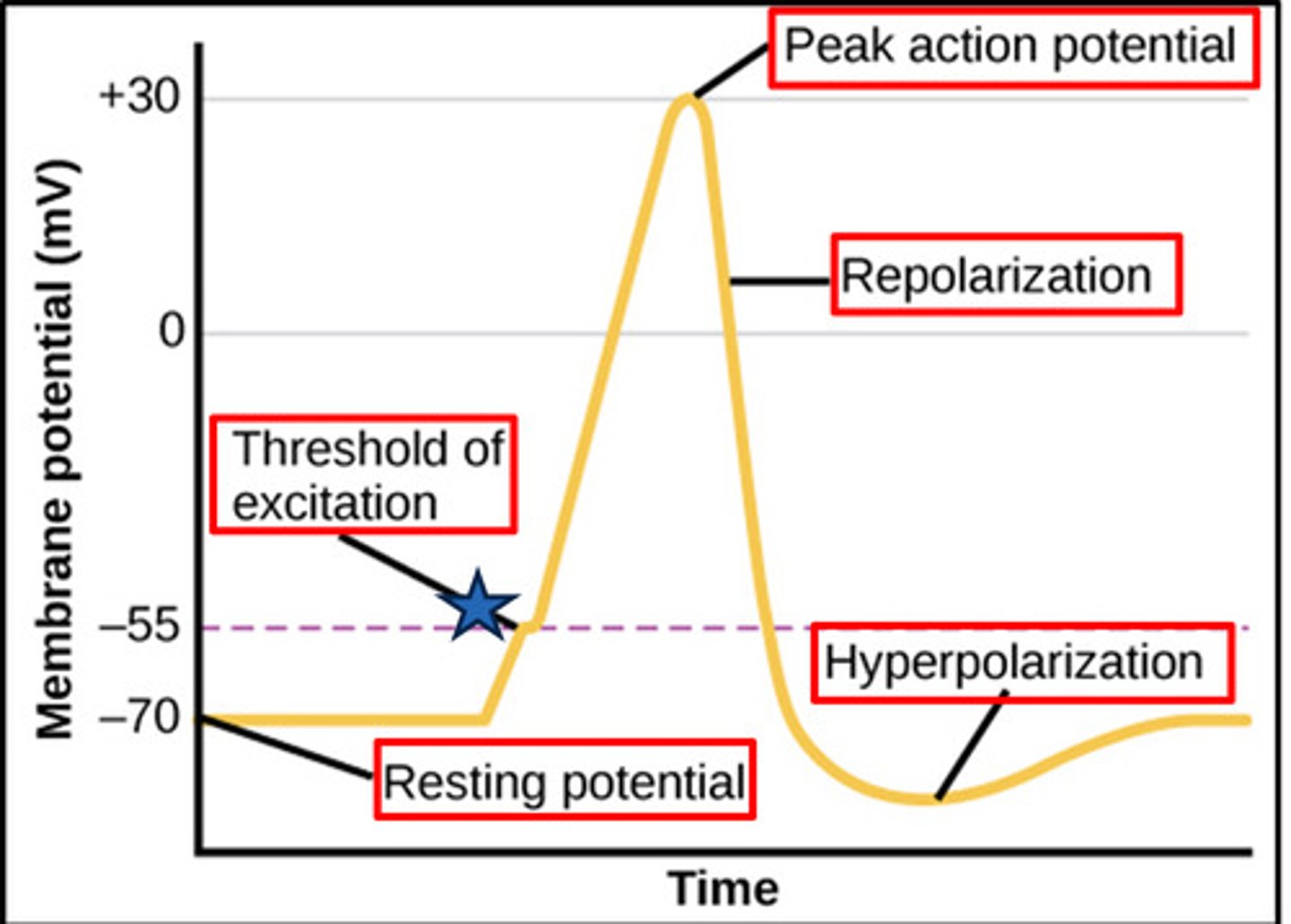
In which phase of action potential is the membrane said to be polarized
resting phase (-70 mV)
Overshoot of nerve fibers in action potential
membrane potential reaches + 35 mV
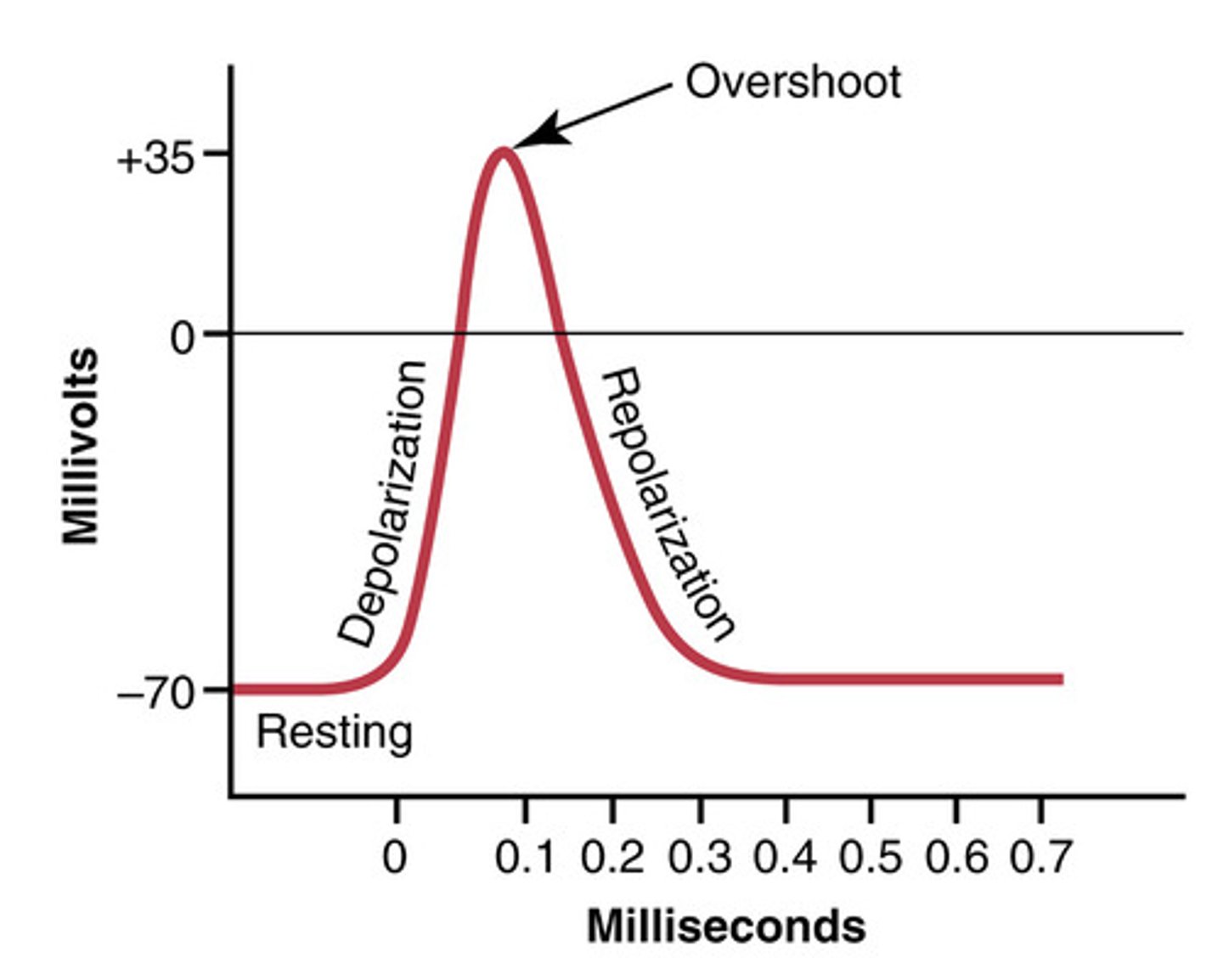
Na channels contain how many gates
two
K channels contain how many gates
one
Resting state Na+ channels (before stimulation)
activation gate closed, inactivation gate open --> channel ready but inactive (-70 mv)
Depolarization rising phase in Na+ channels
membrane potential reaches the threshold (-70 to + 35 mV)
Both gates open --> Na+ rushes into the cell, causing depolarization
Peak/overshoot phase in sodium ion channels
inactivated (+35 to -70 mV, delayed), activation gate open, inactivation gates close (delayed) --> Na+ flow stops
Repolarization phase - Na+ channels
Resting (-70 mV) activation gate closed, inactivation gate open (channel inactive)
Resting state K+ channels
(-70mV), gate is closed at rest
Activation of K+ channels
Slow activation (+35 to -70mV) during depolarization and fully opens in repolarization causing potassium to leave the cell making the inside more negative
Hyperpolarization of potassium channels
K+ channels slow to close --> cause membrane to become more negative (afterpotential) before returning to -70mV
Na+ channel inactivation gates ------ as the channel approaches -70 mV
rapidly reopen
Na+ channel activation gates will be rapidly closed at ------
- 70 mV
Describe the concept of threshold
when depolarization reaches -55 mV, voltage gated Na+ channels open, generating an all or nothing action potential. If threshold isn't reaches no action potential fires
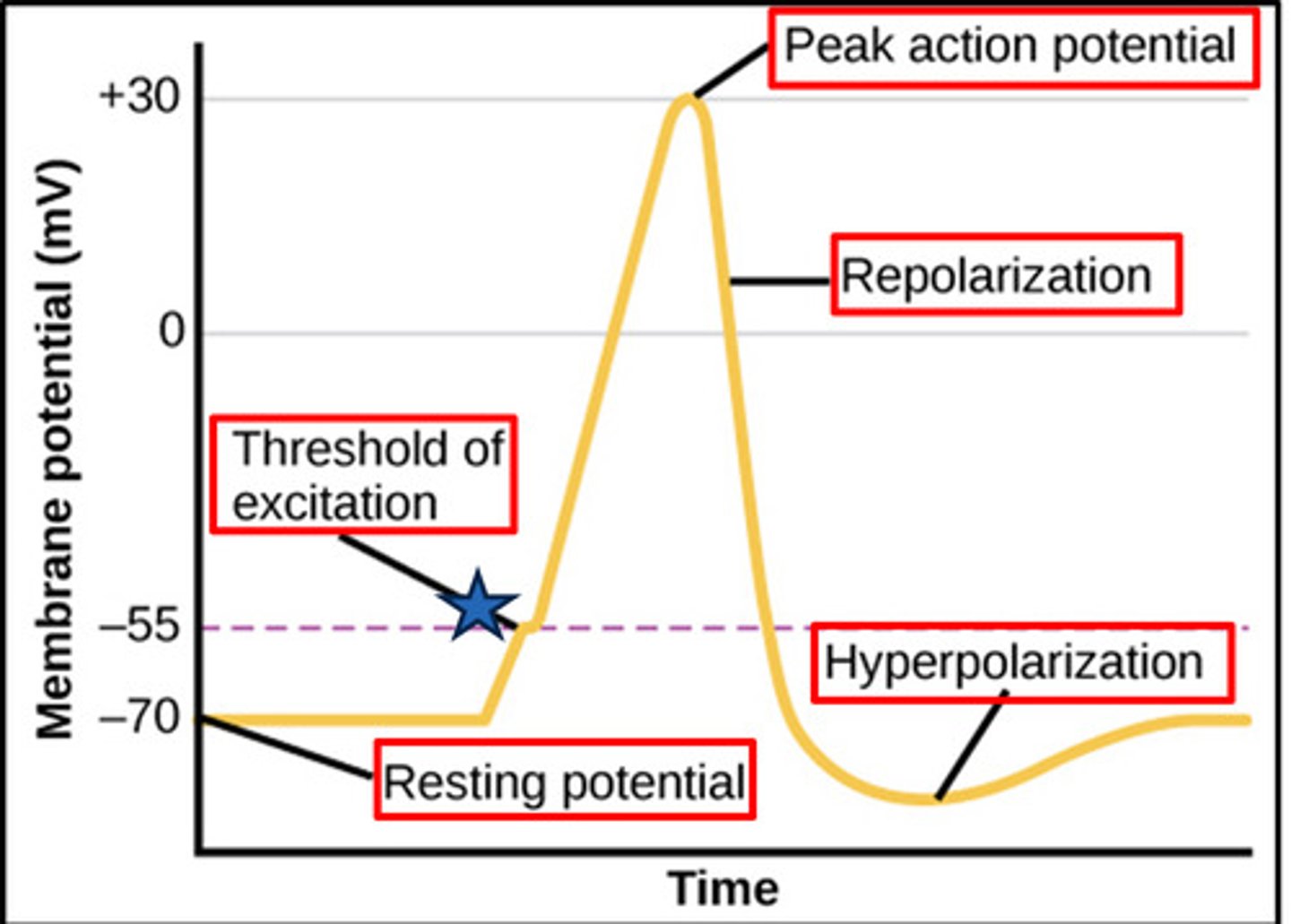
Positive afterpotential tail phase
K⁺ channels remain open longer than needed, allowing excess K⁺ to leave the cell. This drives the membrane potential to undershoot below its resting value (-80 mV to -90 mV) before it returns to -70 mV
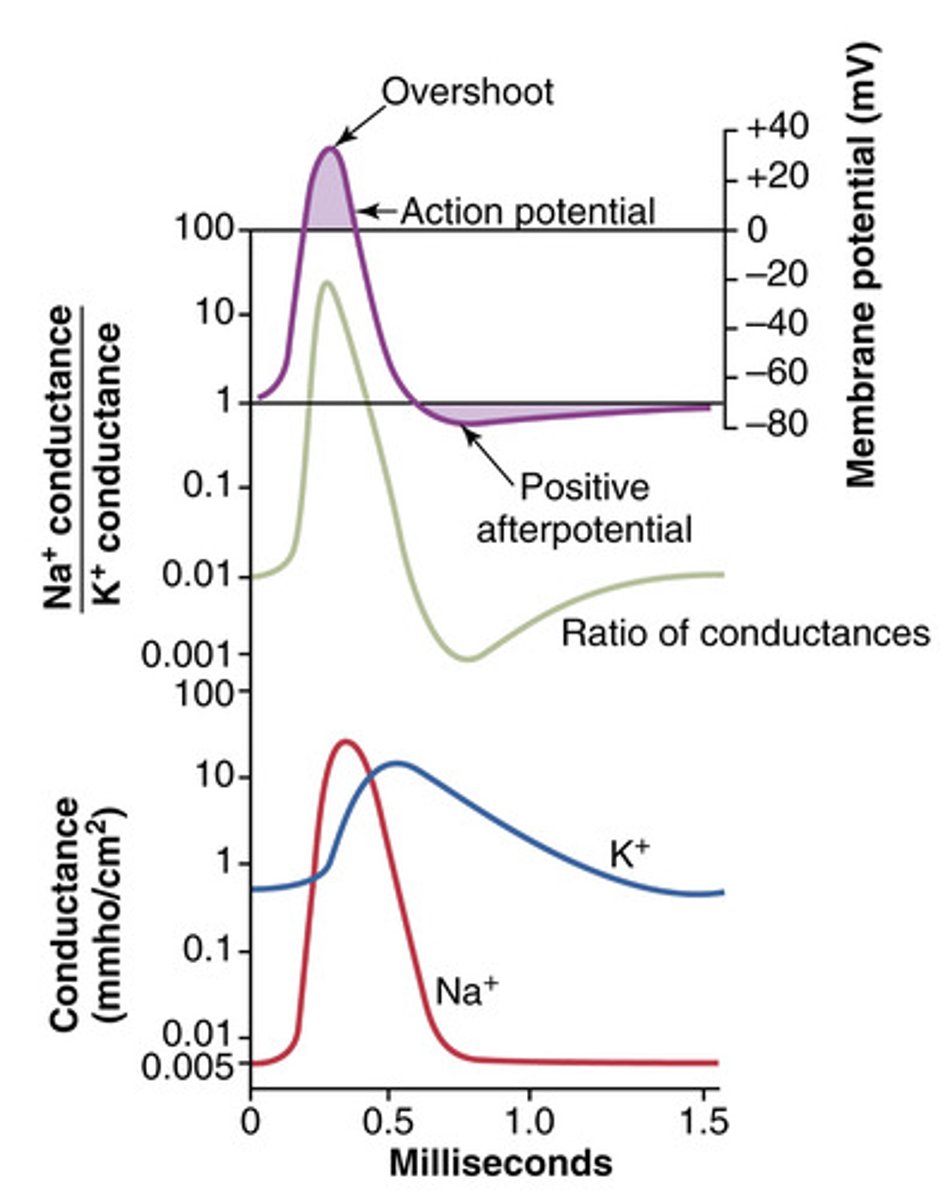
Absolute refractory period
no second action potential can be triggered during this point. Na+ channels are inactivated
Relative refractory
Some Na+ channels reset, a second action potential may be triggered but it must be coupled with an additional larger stimulus.
Structure of an axon
axon hillock, axon fiber, axon terminal, and myelin sheaths
Myelin sheaths
insulate axons, preventing current leak and enabling saltatory conduction
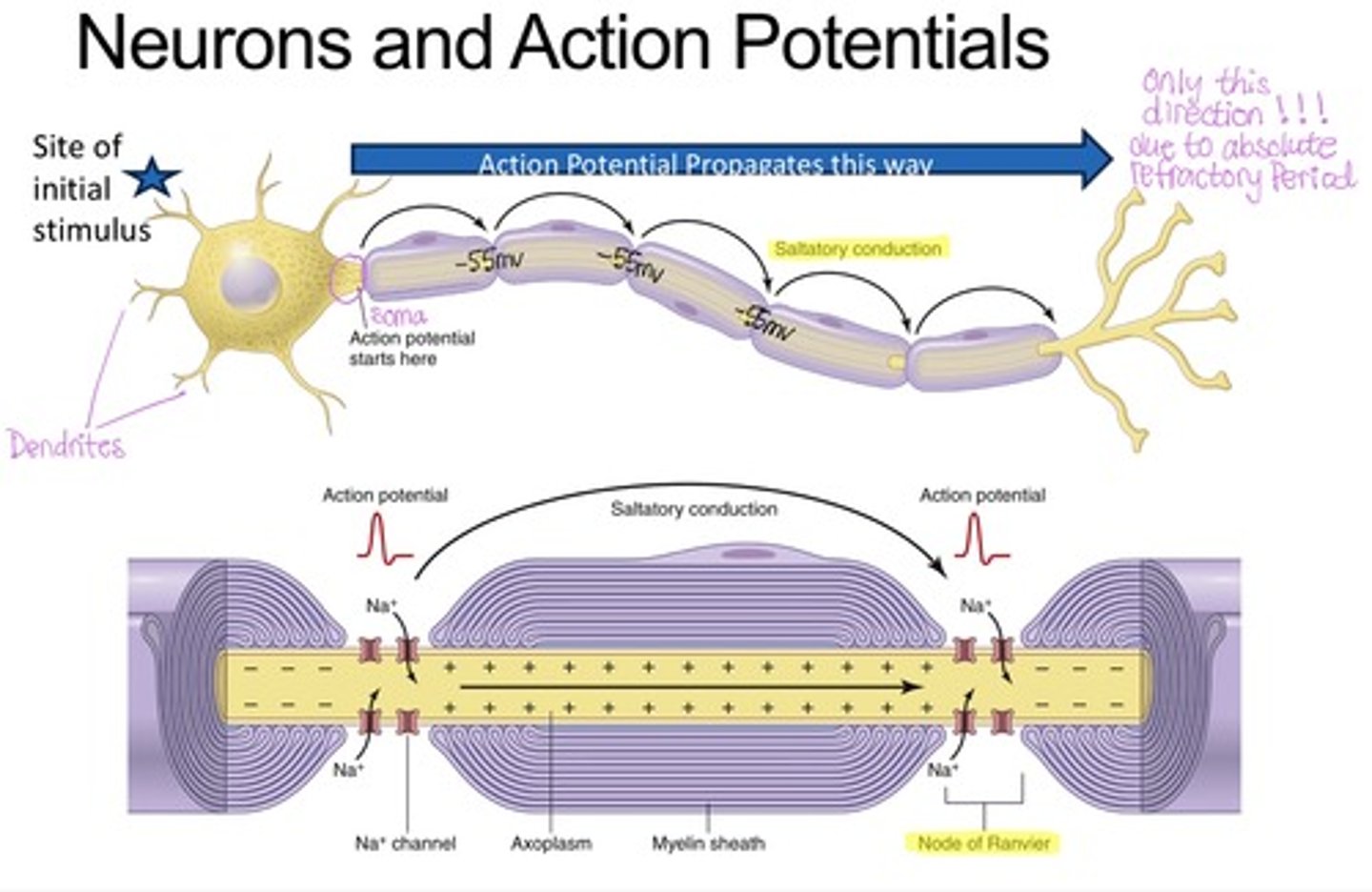
Saltatory conduction
- the action potential propagation along myelinated axons between Nodes of Ranvier, speeding transmission of action potential.
What forms the myelin sheath
Schwann cells
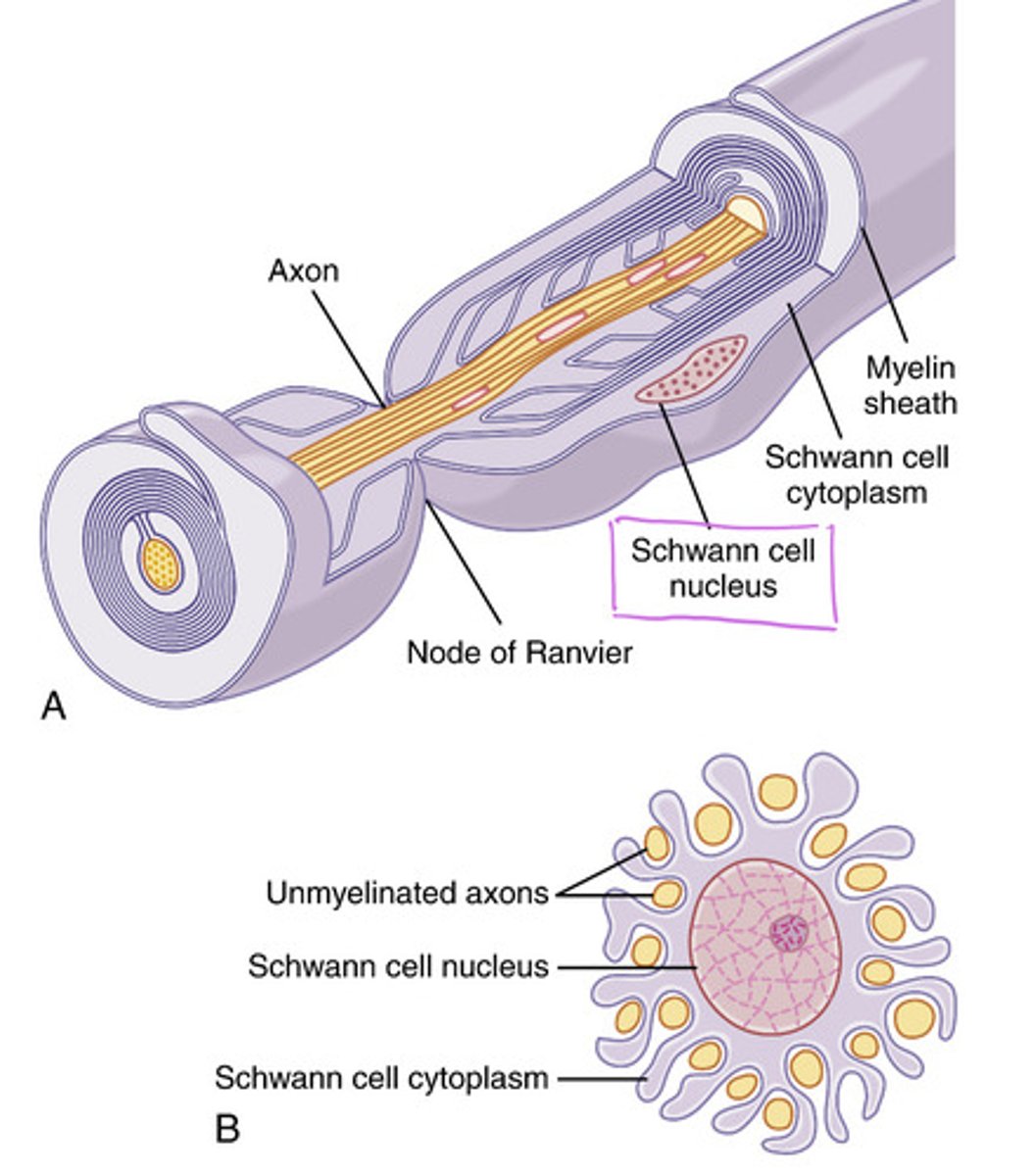
Absent or mutated Schwann cells can cause (clinical correlate)
Multiple sclerosis (MS) --> the action potential would move slowly and occasionally fail to continue
Plateaus mainly seen in
ventricular cardiomyocyte action potential
What causes the plateau phase of cardiac action potential?
result from a balance between Ca2+ influx and K+ efflux -> this maintains the membrane potential near 0 mV for a prolonged period, allowing sustained contraction
What is the physiological importance of the plateau
prolongs depolarization, extends the refractory period, and ensures sufficient Ca2+ entry for cardiac muscle contraction, stops summation
Neurons (nerve cells) contain
dendrites, soma, axon, terminal bouton
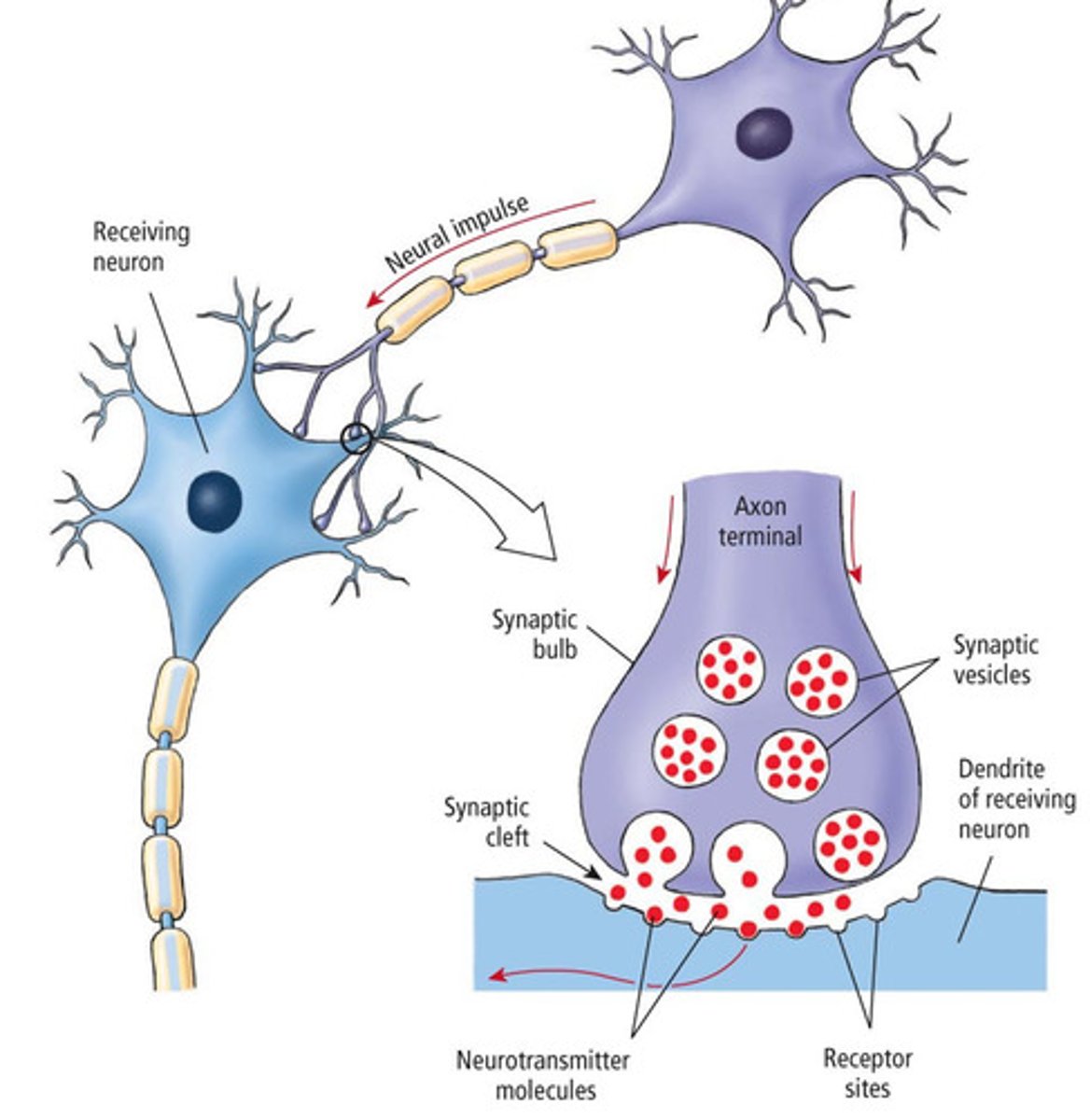
Chemical synapses
neurotransmitters released from presynaptic cells bind to receptors on postsynaptic membrane, most prevalent in the CNS (UNIDIRECTIONAL)
Electrical synapses
Use gap junctions for direct ion flow (between pre and postsynaptic cells) very rapid and BIDIRECTIONAL
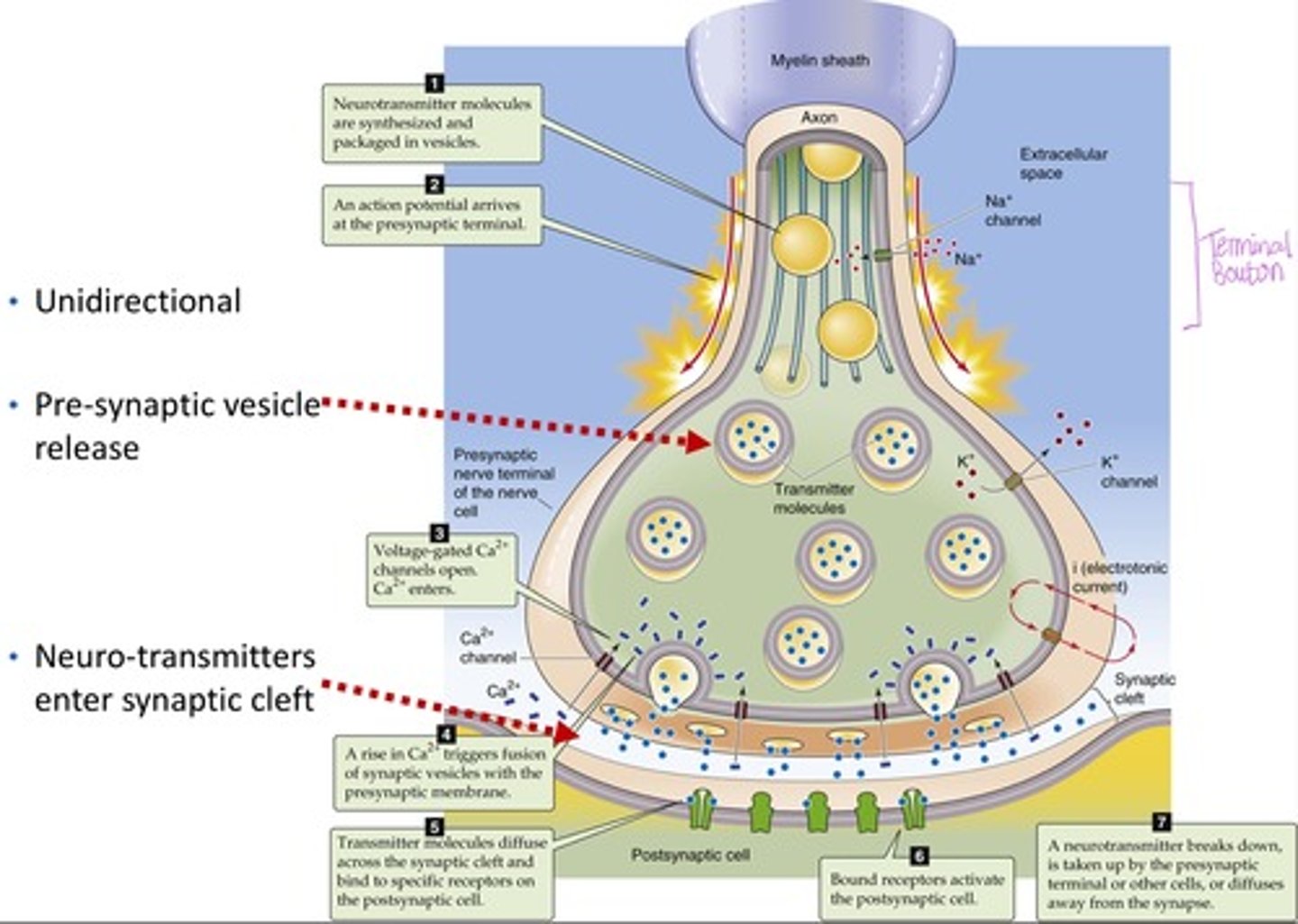
Identify the major steps in synaptic signal transduction
1. Action potential arrives at axon terminal
2. Voltage gated Ca2+ channels open
3. Ca2+ triggers vesicle fusion via v-snare and t-snare
4. Neurotransmitter released into synaptic cleft
5. Neurotransmitter binds to postsynaptic receptors, generating EPSPs or IPSPs
6. Signal terminated by degradation, reuptake, or diffusion.
What do neurotransmitters generate when they bind to postsynaptic receptors?
EPSPs or IPSPs
How is the synaptic signal terminated?
degradation, reuptake, or diffusion
What is a neurotransmitter
messenger of neurologic information from one cell to another
Small molecule transmitters (fast or slow, where are they synthesized and absorbed)
- Rapid acting
- Mostly synthesized in the cytosol of presynaptic terminal and absorbed via active transport into vesicles
Examples of small molecule transmitters
- acetylcholine
- amines; dopamine, norepinephrine, epinephrine, serotonin, histamine
- amino acids; glutamate, GABA, Glycine, Aspartate
- nitric oxide
Neuropeptides (fast or slow, cause and effect)
- Slow synthesis but more potent and prolonged effect than small mol.
- Causes prolonged closure of Ca2+ channels, txn changes and effect may last days - years
Neuropeptides classes
- Hypothalamic releasing hormones
- Pituitary peptides
- Peptides that act on gut and brain
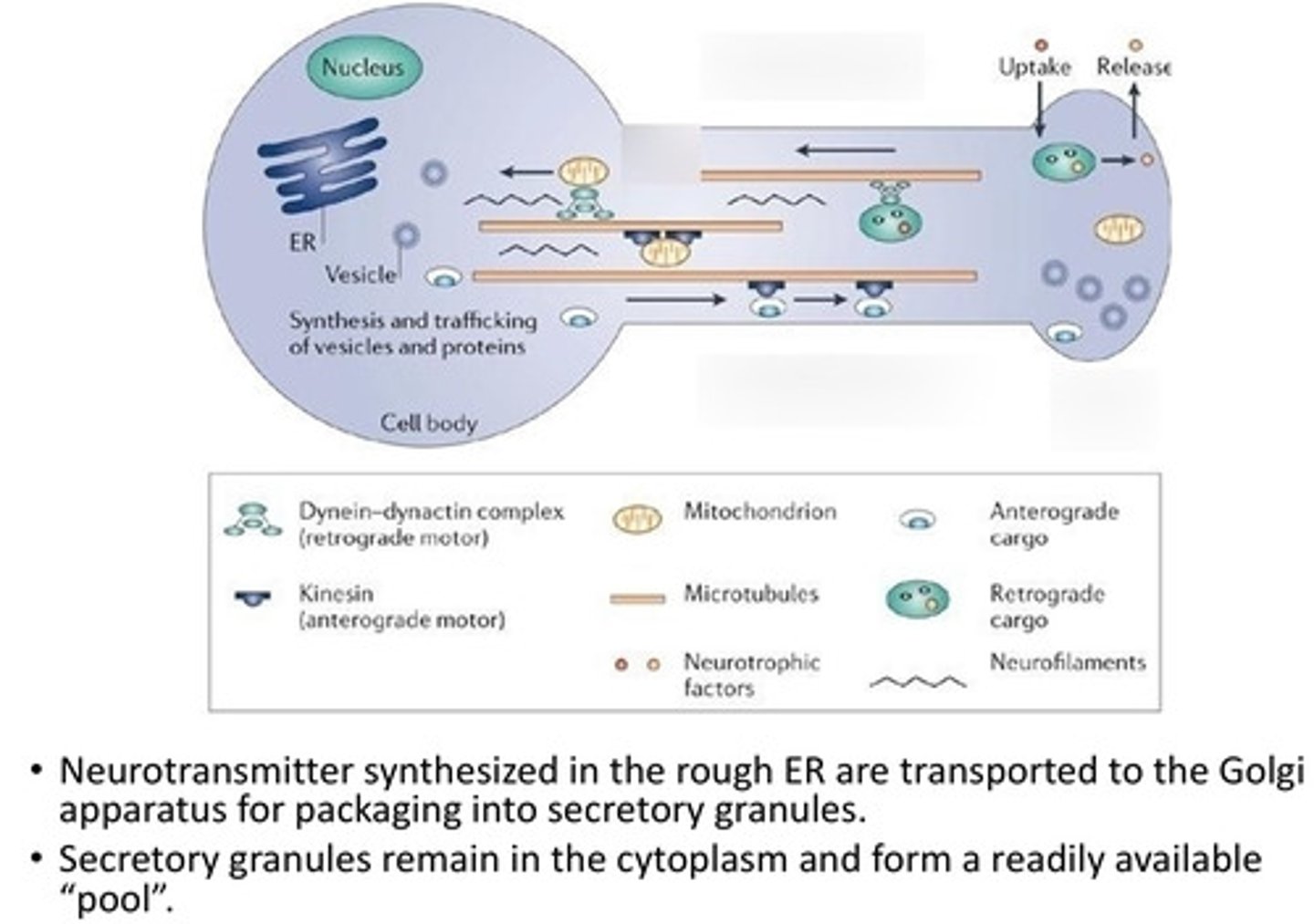
Where are neurotransmitters synthesized and transported?
ER and transported to the golgi for packaging into secretory granules
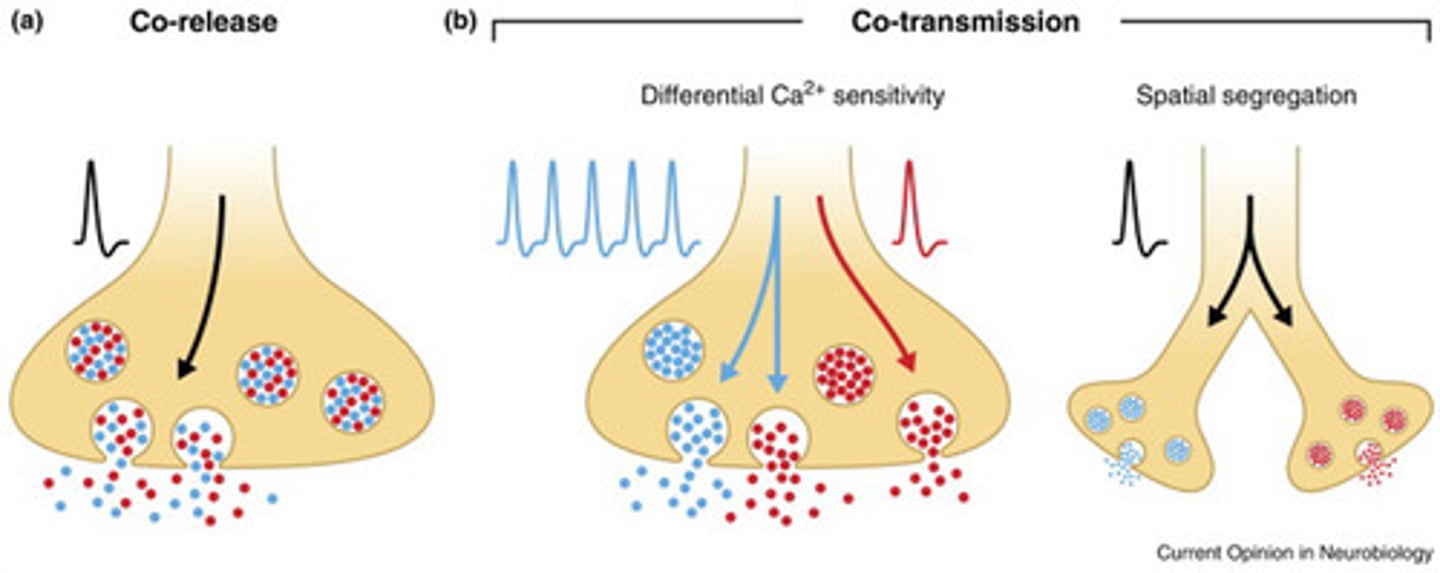
Co-release of neurotransmitters
co-localized in the same synaptic vesicles and released together
Co-transmission of neurotransmitters
localized in different vesicles and may be differentially regulated because of different calcium ion sensitivities or because they are located in different boutons
V-SNARE made up of
structural protein
- synaptotagmin
- synaptobrevin
T-SNARE made up of
- Syntaxin
- SNAP-25
Binding of v-snare and t-snare requires
Calcium
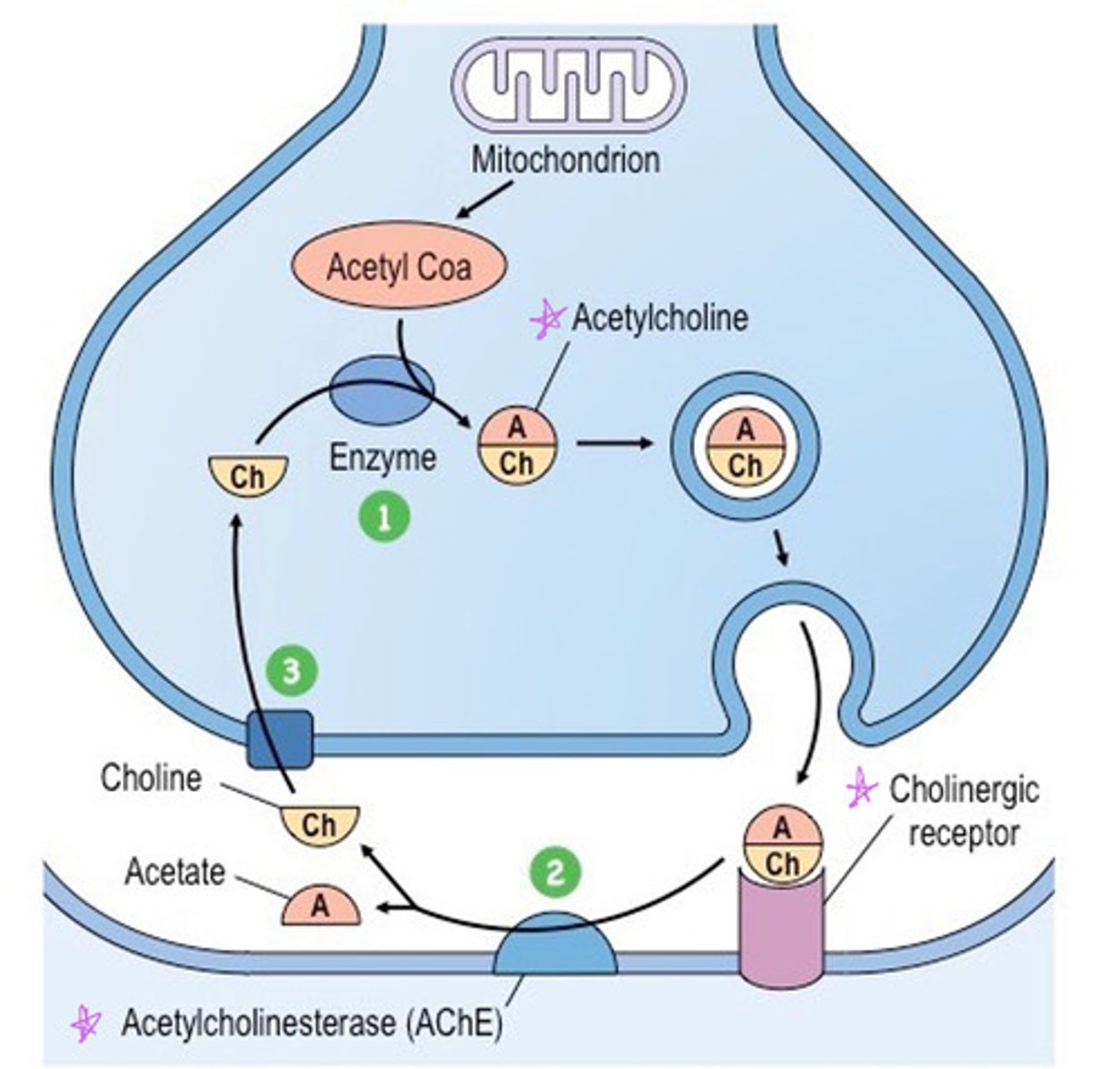
Acetylcholine is made from
choline and acetyl CoA
Acetylcholine binds to
cholinergic receptors in the synapse
In the synapse, ACh is rapidly broken down by the enzyme ____
acetylcholinesterase (AChE) and transported back into the axon terminal to make more Ach
Glutamate is an ______ neurotransmitter
excitatory
where is glutamate found
Widely distributed in the CNS
Glutamate binds glutamate receptors on postsynaptic membrane causing opening of which channels
Na+ and Ca2+ signals next cell to have an action potential
Glycine neurotransmitter
major inhibitory neurotransmitter in spinal cord and brainstem
Glycine binds to glycine receptors on the postsynaptic cell causing opening of which channel
Cl- channel which signals next cell to NOT have an action potential
Biogenic amines
dopamine, norepinephrine, epinephrine (Think Do Not Escape)
Which enzymes degrade dopamine, norepinephrine, and epinephrine?
MAO (monoamine oxidase) and COMT
Ionotropic receptors
Ligand-gated ion channels--fast response (NT: Ach, glutamate, glycine)
Metabotropic receptors
receptors that are associated with signal proteins and G proteins-- slower, longer-lasting effects
Example of ionotropic receptor
Nicotinic ACh receptor
Example of metabotropic receptor
Muscarinic ACh receptor
Excitatory postsynaptic potential (EPSP)
depolarizing, increases the likelihood of action potential firing (Ach, glutamate)
Inhibitory postsynpatic potential (IPSP)
hyperpolarizing, decreases the likelihood (GABA, glycine)
Neuronal summation
Neurons integrate all excitatory and inhibitory inputs in the soma. If the net depolarization at the axon hillock reaches threshold, an action potential fires.
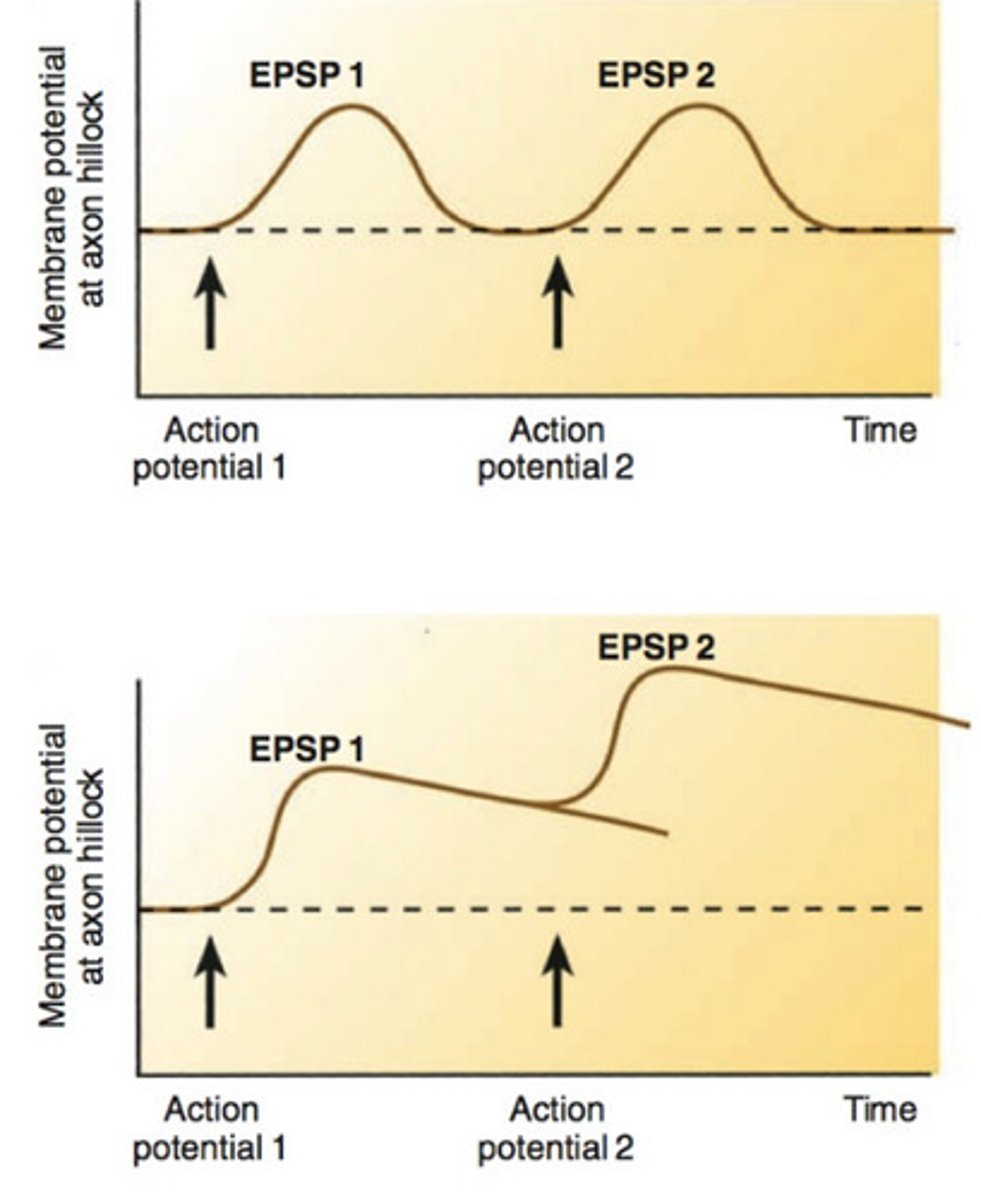
Temporal summation
One or more presynaptic neurons transmit impulses in rapid-fire order. (if enough EPSPs occur close together, they can reach threshold and trigger action potential)
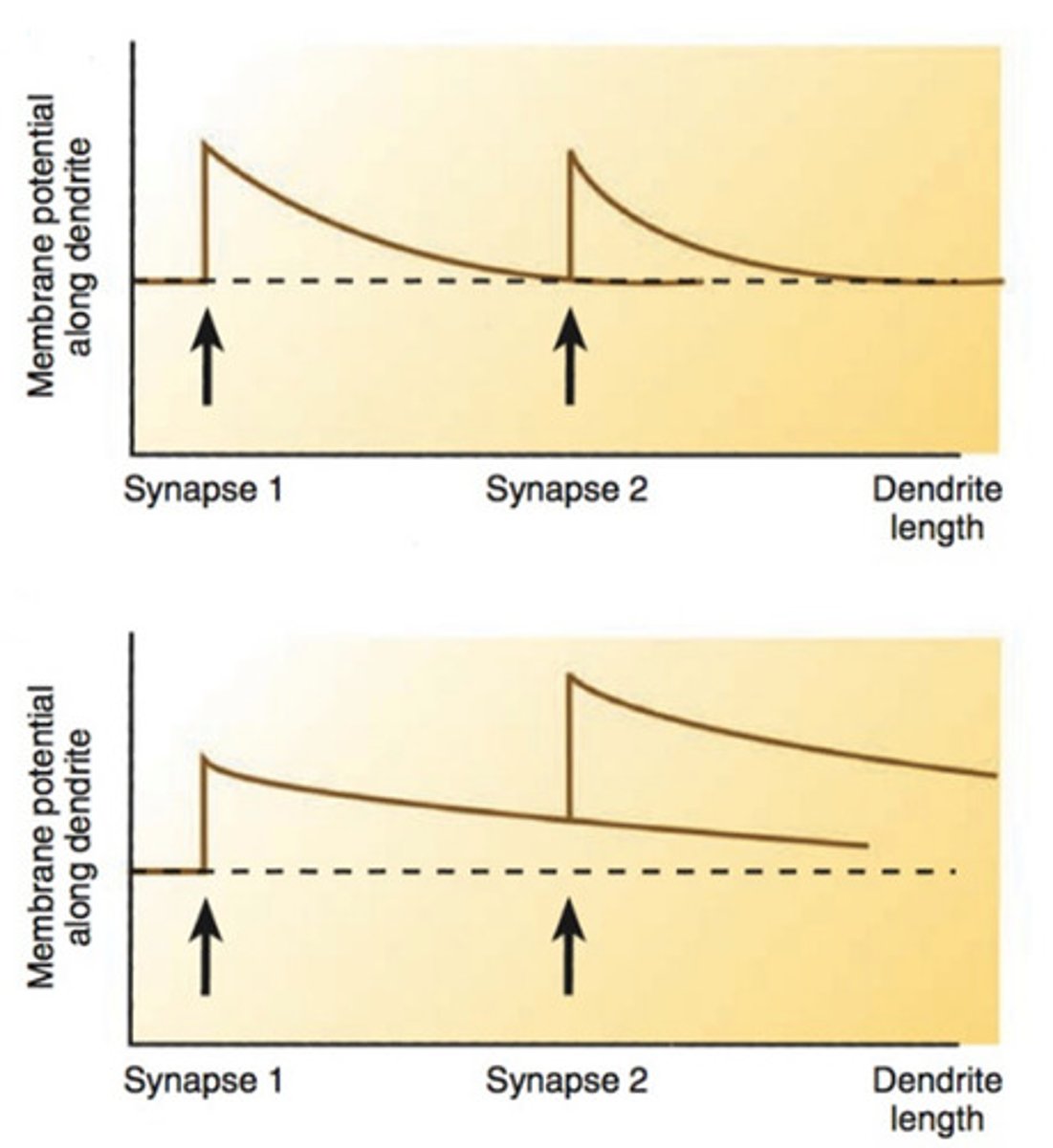
Spatial summation
think multiple presynaptic neurons firing at the same time, each at a different location on the dendrites --> can add up in space and reach threshold (fire action potential)
What is the excitatory state
Excitation is greater than inhibition --> causes neurons to fire repeatedly as long as that state is maintained.