Lecture 3 GLUT, NADH, TCA cycle, ETC and oxidative phosphorylation
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
97 Terms
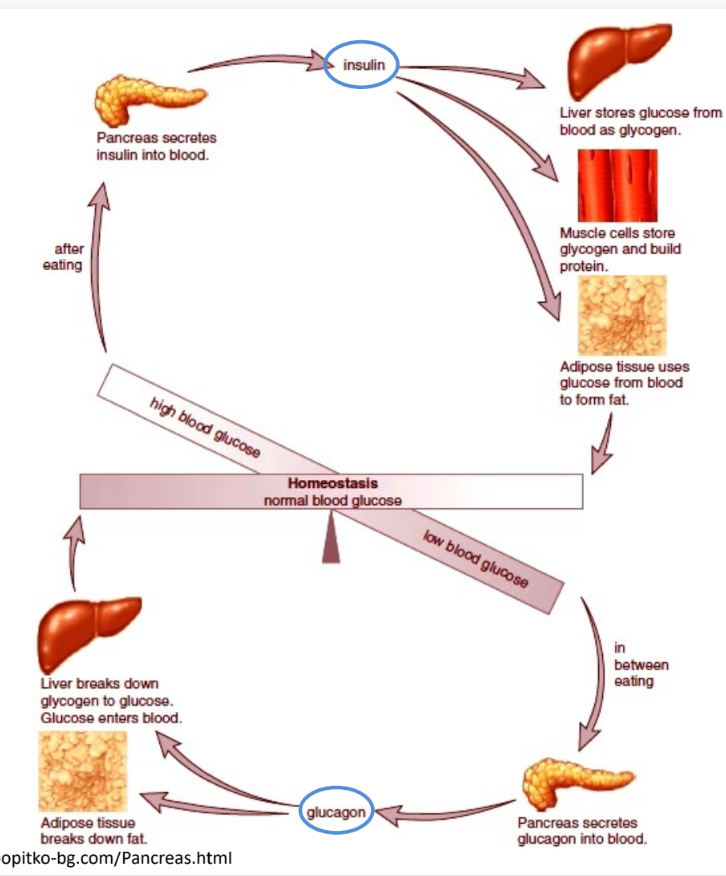
Key takeaways
when you eat meal, blood sugar levels increase
insulin released so that liver stores glucose as glycogen
blood sugar levels maintained
if too low, glucagon released to tell liver to break glycogen into glucose
homeostasis maintained
GLUT
Glucose Transport Proteins
GLUT1
ubiquitously distributed, red blood cells; constitutive transport
GLUT2
intestine, liver, kidney, pancreas
GLUT3
CNS, brain
GLUT4
skeletal muscle, adipose tissue, heart (insulin-regulatable)
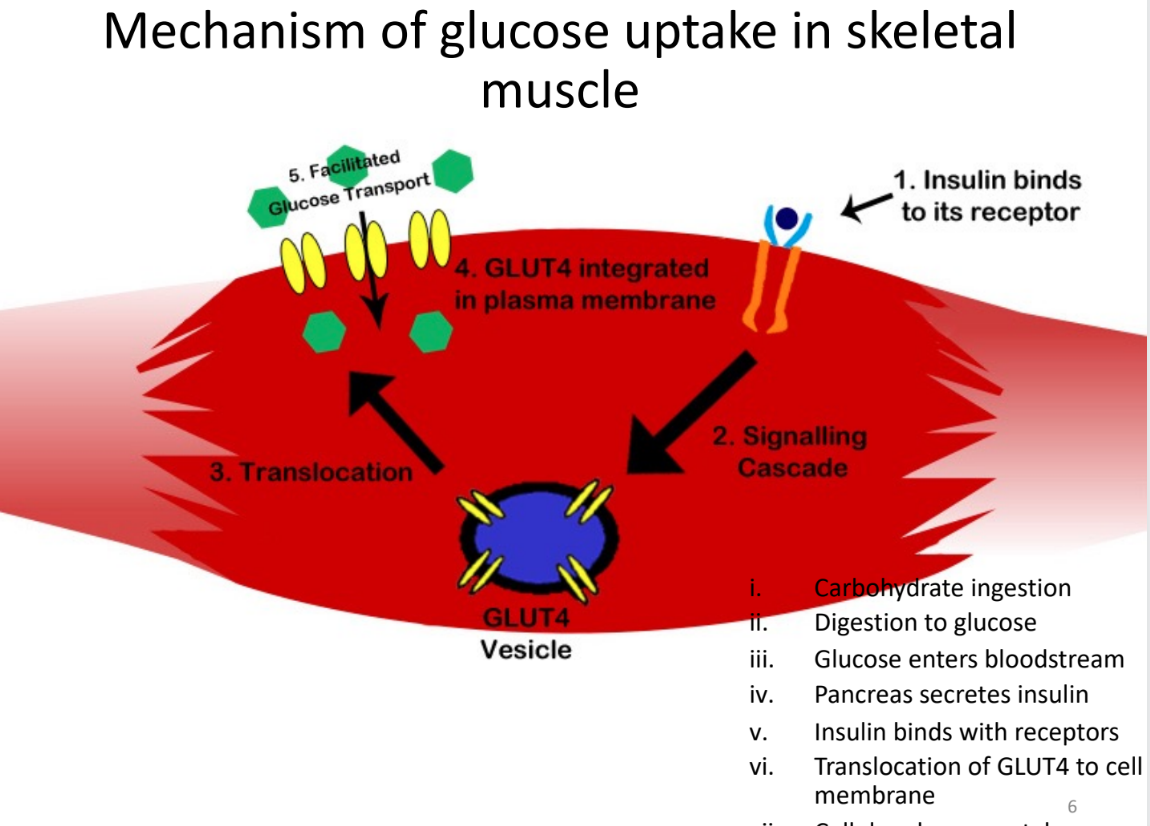
Mechanism of glucose uptake in skeletal muscle
insulin binds to its receptor
signalling cascade releases GLUT4 vesicle
translocation
GLUT4 vesicle integrated in plasma membrane
facilitated glucose transport
oxidation of glucose
glucose + 6O2 = 6CO2 + 6H2O
glycolysis partial oxidation of glucose net equation
glucose + 2pi + 2ADP + 2NAD+ = 2ATP + 2NADH + 2 pyruvate + 2H+ + 2H2O
NADH is
reducing equivalent
reducing equivalents
carry electrons that can be transfered btw molecules
coenzymes that act as electron carriers
FAD, NAD+ and NADP
electron carriers are reduced by
adding electrons
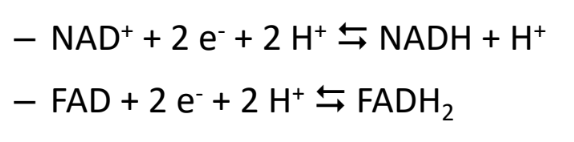
NAD+ is derived from
vitamin B3
FADH2 and NADH are generated from
oxidation (ex. glycolysis, beta oxidation, TCA cycle)
anabolism
synthesis
reductive bc gain to build
reducing equiv NADPH & ATP
catalyzed by reductases
catabolism
degradation
oxidative bc loose to break
makes reducing equiv NADH, FADH2, ATP
via dehydrogenase
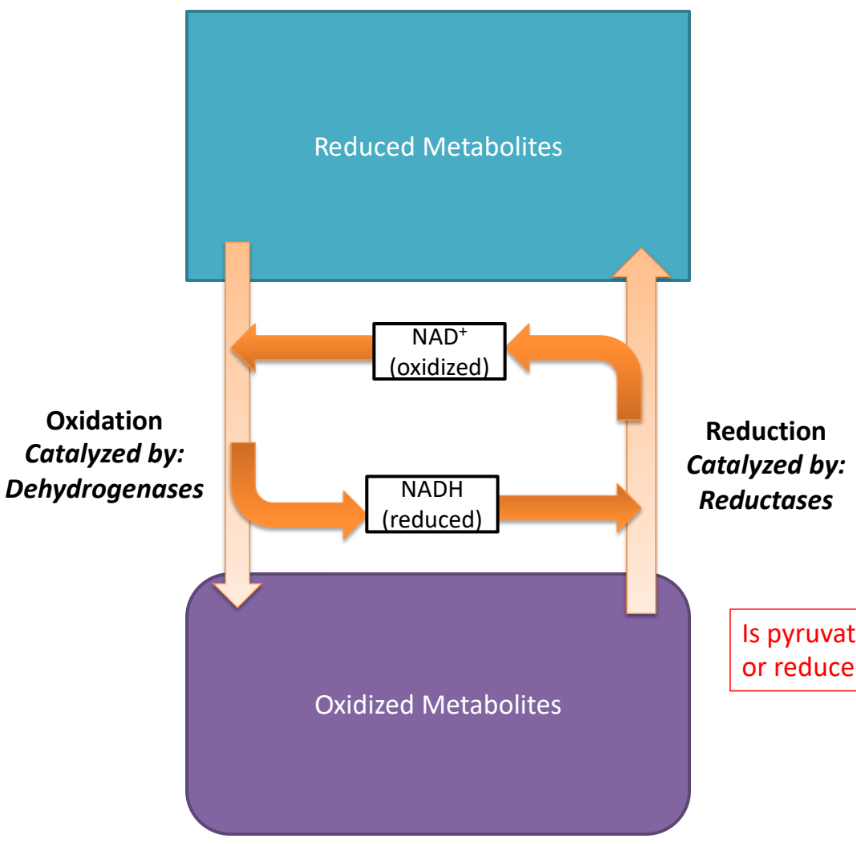
pyruvate is more ____ than glucose
oxidized
NAD+ is ___ form
oxidized bc lost electrons
NADH is ___ form
reduced bc has elextrons
obligate aerobes
Grow only in presence of oxygen
Facultative anaerobes
Can grow with or without oxygen
annelids, mollusks and some yeast
obligate anaerobes
Cannot grow in oxygen and metabolize glucose only anaerobically
lactic acid bacteria
used for fermenting yogurt, cheese
generate ATP by fermentation of sugars to lactate
yeast
converts pyruvate to ethanol in wine- and beer-making
after glycolysis
complete oxidation of glucose in mitochondria
mitochondria
oxidize metabolic fuels to generate energy
similar to power plants
biological oxidations are catalyzed by enzymes
pyruvate is oxidized by
pyruvate dehydrogenase
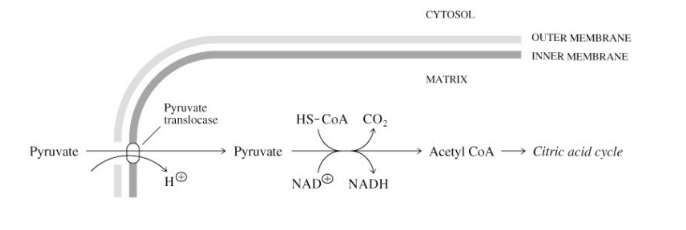
Pyruvate is transported into the mitochondria via
pyruvate-H+ symport
pyruvate dehydrogenase catalyzes
irreversible oxidative decarboxylation of pyruvate to acetylCoA
Pyruvate (3C) + CoA + NAD+ =
acetylCoA (2C) + CO2 (1C) + NADH
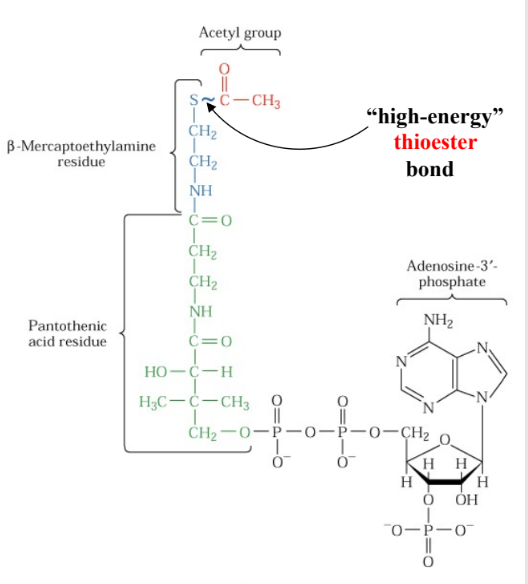
CoA
coenzyme A
derivative of vitamin B
pathogenic acid (rich in poultry, yogurt, avacado)
acts as carrier
bond btw acetyl and CoA is
high energy thioester bond
entry of acetyl CoA into TCA completes
oxidation of glucose to CO2
TCA enzymes are
compartmentalized
soluble in matrix EXCEPT succinate dehydrogenase (membrane protein)
TCA equation
3NAD+ + FAD + GDP + Pi + acetylCoA = 3 NADH + FADH2 + GTP + CoA + 2CO2
TCA cycle beings and ends with
oxaloacetate
product of first reaction in TCA =
citrate = tricarboxylic acid
step 1
simps
Citrate Synthase
step 2
ask
Aconitase
step 3
dumb
Isocitrate Dehydrogenase
step 4
daughters
A-ketoglutarate Dehydrogenase
step 5
sucking
Succinyl-CoA Synthase
step 6
d*ck
Succinic Dehydrogenase
step 7
for
Fumerase
step 8
drinks
Malate Dehydrogenase
summary of TCA
First step combines 4-C oxaloacetate with 2-C acetylCoA to form a 6-C
compoundFirst two dehydrogenase reactions are much like PDH, producing NADH
and removing 2 CO 2 moleculesThe third dehydrogenase reaction produces FADH 2
The final step of the cycle is the fourth dehydrogenase that produces the
third NADHThe first reaction is catalyzed by a synthase (no ATP involved) whereas
the fifth reaction is catalyzed by a synthetase (involves ATP/GTP being
used or synthesized, like a kinase)
glucose oxidation (start to finish)
Glucose has now been completely
oxidized to CO 2Electrons removed from glucose by 6 dehydrogenases were transferred to
reducing equivalents
• Recall that 2e - are required to reduceNADH and FADH 2 become reoxidized by
passing their electrons to the ETC
mitochondria anatomy
Outer membrane (OM) encapsulates
the mitochondrionInner membrane (IM) is invaginated,
forming cristae
cristae
increase the surface area of
the inner membrane
inner membrane
greater amount of
protein than OMproteins of ETC,
oxidative phosphorylation and
transport proteinsNumber of cristae varies with
oxidative capacity of the tissue
(liver has very few vs muscle and heart have more)
matrix
Contains PDH, enzymes for TCA cycle, β-
oxidation and amino acid oxidation,
mitochondrial DNA# of mitochondria varies (Exercise training increases number of
mitochondria in muscle)
outer membrane is permeable via
porin
Inner membrane is relatively impermeable
Permeable to only to small uncharged compounds O2 , CO2 , H2 O
Contains a variety of transport proteins (translocators) for ATP, ADP, pyruvate, Pi , H+ which are all charged and very hydrophilic
*relative impermeability creates conc gradient
transport systems required for inner membrane for transport of
ATP
2. ADP + Pi
3. NADH electrons
ATP
(produced in the mitochondria) into the cytosol to be used by ATP-consuming processes
ADP + Pi
generated from ATP utilization in the cytosol) into the
mitochondria to be resynthesized into ATP by oxidative phosphorylation
NADH
(generated from glycolysis in the cytosol) into the mitochondria for ETC
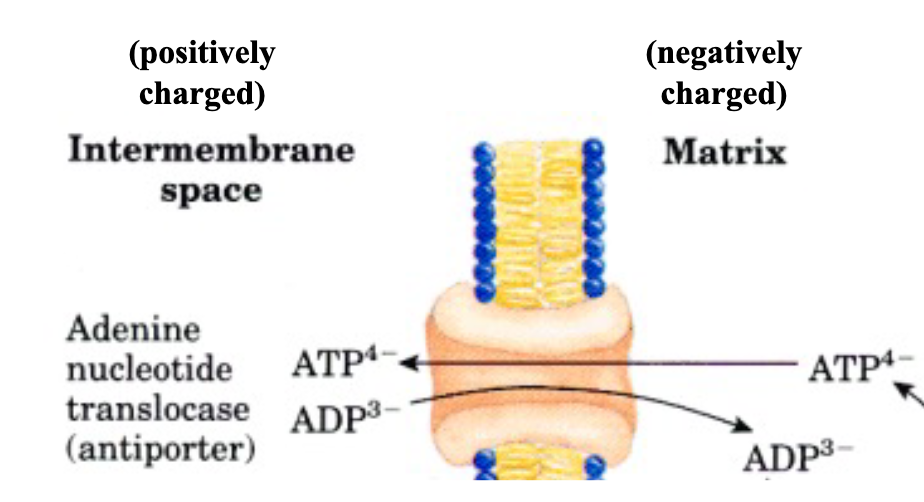
ADP-ATP Translocator
Exports ATP to cytosol in exchange for ADP (ATP out of matrix, ADP into matrix)
Driven by membrane potential difference
drives ATP outside of mitochondria since since ADP
has 3 –ve charges and ATP has 4 –ve charges
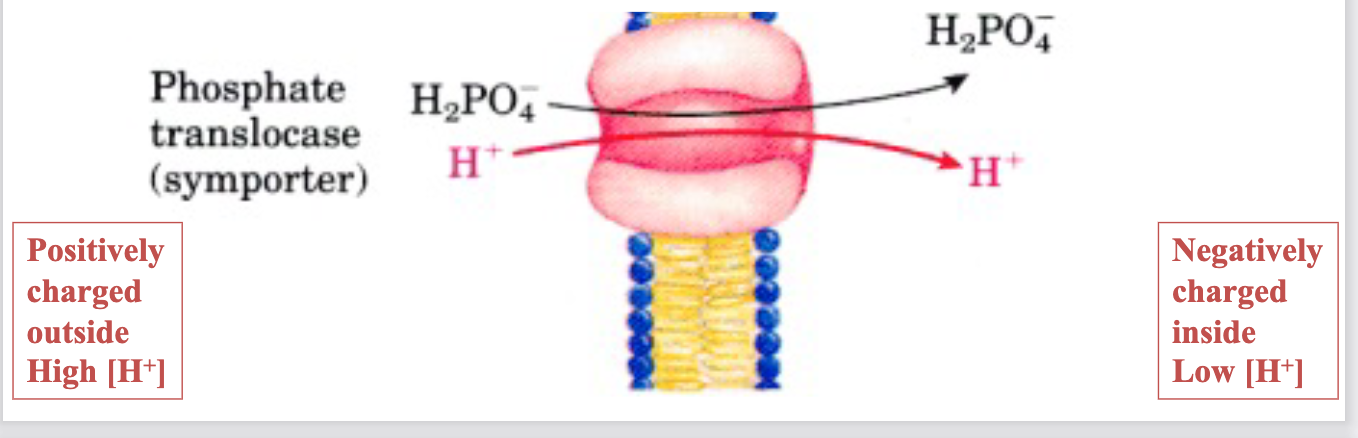
P i -H + translocase
P i (H 2 PO 4- ) generated from ATP hydrolysis is transported into the
mitochondria via a symporter along with H +Driven by the electrochemical gradient of H +
proton gradient drives H + to equilibrate, by H + entering the mitochondria
via symport with P i (electroneutral)
Malate-aspartate shuttle: transport of NADH electrons
cell maintains separate pools of NADH in mitochondria and cytosol
NADH generated in the cytosol by glycolysis transfers electrons into the
mitochondria via malate intermediate in a very complex transport
mechanism
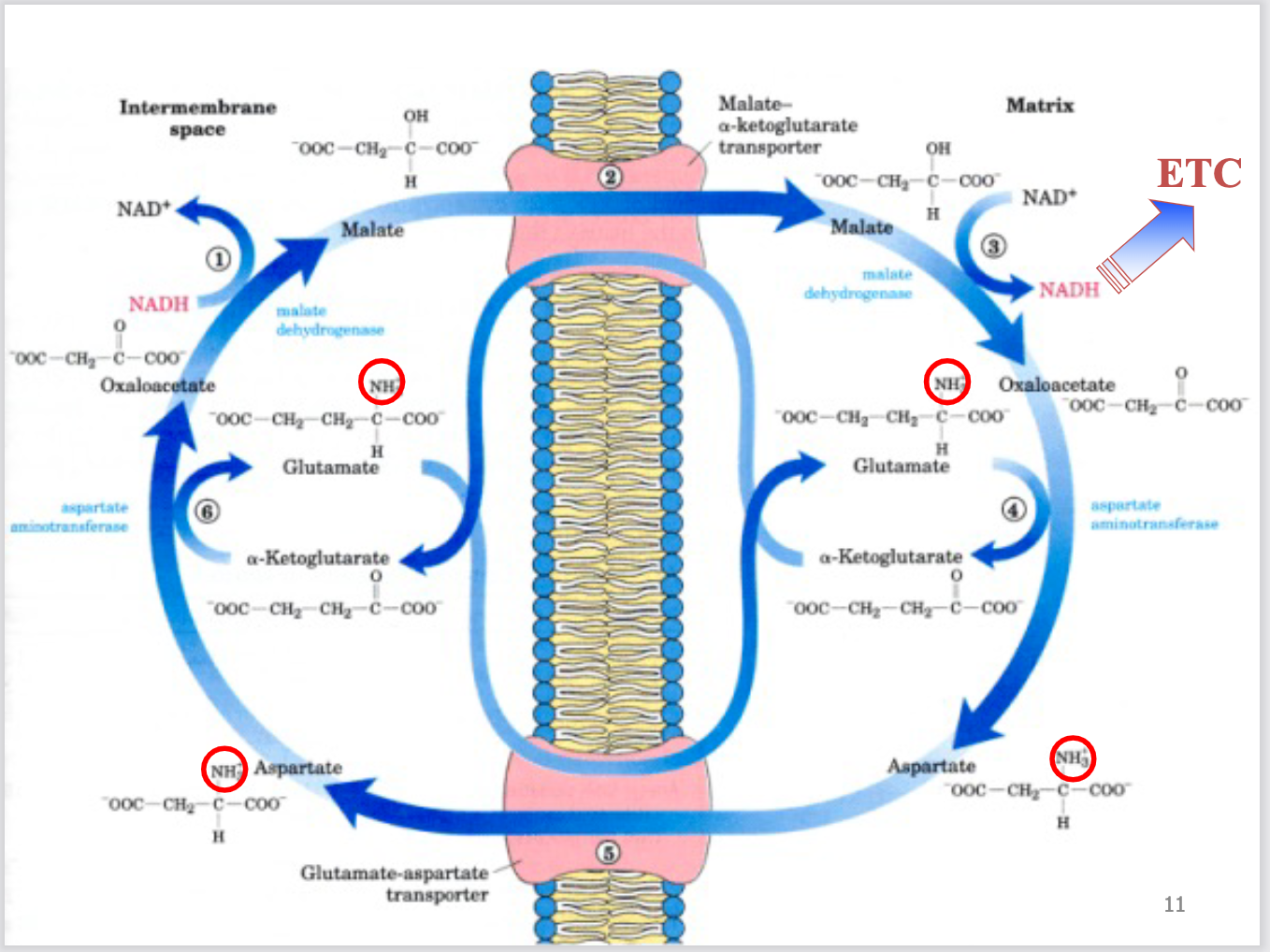
Malate-aspartate shuttle: transport of NADH electrons
e - s donated from NADH to oxaloacetate to yield malate by cytosolic
malate dehydrogenase
2. Malate is transported into mitochondria by malate-α-ketoglutarate
transporter
3. Malate reoxidized to oxaloacetate, transferring e - s to NAD+ by
mitochondrial malate dehydrogenase, yielding NADH in the
mitochondria for ETC
4. Oxaloacetate cannot cross the IM, \is converted to aspartate by
mitochondrial aspartate aminotransferase (transfers amino group)
5. Aspartate is transported into cytosol by glutamate-aspartate
transporter
6. Aspartate reconverted to oxaloacetate by cytosolic aspartate
aminotransferase
malate aspartane shuttle 2 enzymes
separate mitochondrial and cytosolic pools
1.malate dehydrogenase (1 & 3)
2. aspartate aminotransferase (4 & 6)
malate aspartane shuttle 2 transporters
to exchange aspartate and α-ketoglutarate for
oxaloacetate/aspartate conversion
1.malate-α-ketoglutarate transporter (2)
2. glutamate-aspartate transporter (5)
glucose has been oxidized to co2 via
glycolysis pyruvate dehydrogenase, TCA cycle
ETC
Composed of 4 protein complexes in inner mitochondrial
membrane and two carrier proteins
– Complexes I, II, III, IV
– Coenzyme Q (Q) and Cytochrome c (Cyt c
final electron acceptor
oxygen
2 functions of protein complexes 1-4
shuttle electrons
pump protons
shuttle electrons
Through redox centres with progressively greater reduction
potential
– Proteins themselves are not reversibly reduced/oxidized
– Complexes contain combination of two or more redox centers
• Coenzymes
• Fe-S clusters
• Cytochromes
• Cu
pump protons
By harnessing the free energy released from electron transport
• Complex I – accepts electrons from NADH and pumps 4 protons
• Complex II – accepts electrons from FADH 2 (does not pump!)
• Complex III – pumps 4 protons
• Complex IV – pumps 2 protons
20
Complex I
accepts electrons from NADH and pumps 4 protons
Complex II
accepts electrons from FADH 2 (does not pump!)
Complex III
pumps 4 protons
Complex IV
pumps 2 protons
NADH uses
complex I, III, IV
FADH2 uses
complexes II, III, IV
complex II contains
TCA enzyme succinate dehydrogenase
FAD is
prosthetic group covalently bound to succinate
*As FADH 2 is formed by succinate dehydrogenase it is readily
reoxidized by Complex II
complexes I, III, IV uses
free energy of electron transport to pump H+ from matrix to intermembrane space
this generates electrochem gradient across inner memb
oxidative phosphrylation
potential energy in the electrochemical gradient of protons is coupled to
oxidative phosphorylation of ATPfree energy released upon re-entry of protons into the matrix is
harnessed by ATP synthase to drive the phosphorylation of ADP
peter d mitchhell nobel prize winner
ubiquinone and proton pump
F 1 F 0 -ATPase
made up of two functional domains held together by a protein stalk
F1 + protein stalk + F0
F1
contains ATP synthetase enzyme
protein stalk
binds F1 to F0
F0
proton channel that spans IM
complexes I and III pump
4H+
complex IV pumps
2H+
complex II
electron transfer does not provide enough energy to pump protons
NADH pumps
10 protons
yielding 3 ATP ratio
FADH2
pumps 6 protons
yeilding 2 ATP
partial oxidation of glucose through glycolysis yields
2 ATP
complete oxidation of glucose yeilds
38 ATP