Cancer Biology exam 2 Uark
1/80
Earn XP
Description and Tags
Yuuchun Du
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
81 Terms
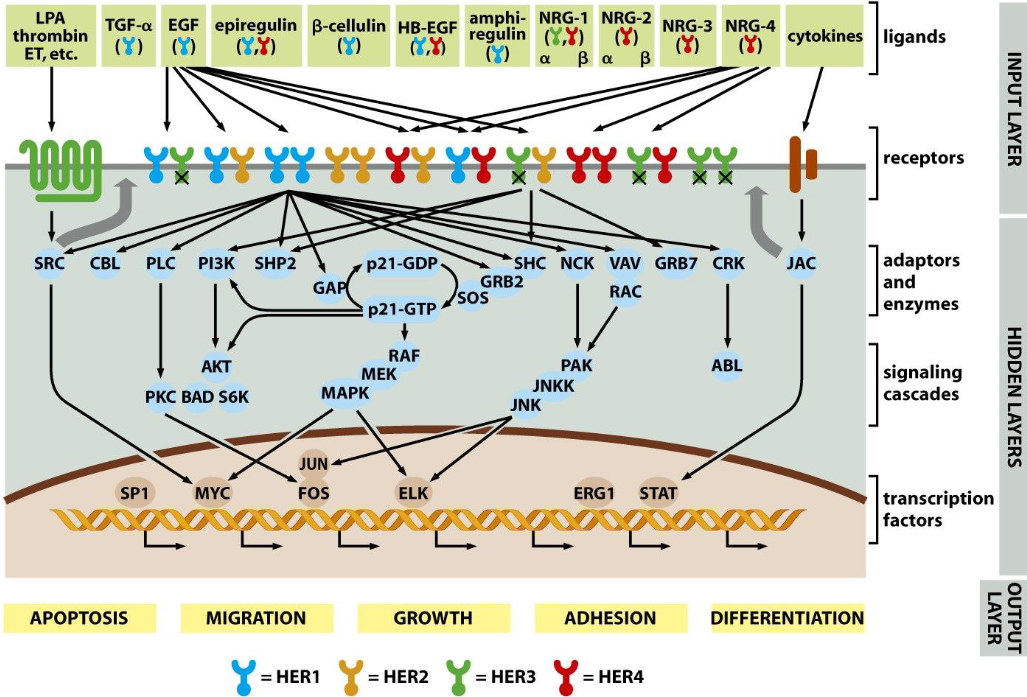
How do cells communicate with their surroundings?
Ligands→ Receptors→ adaptors and enzymes→ signaling cascades→ transcription factors
Normal metazoan cells control each other’s lives:
➢ In order to create normal tissue structure and function, the different cell types must coexist.
➢ The relative numbers and positions of each cell type must be tightly controlled.
➢ The control is largely achieved via the exchange of signals between cells within a cell type, and between different cell types.
➢ All the decisions made by an individual cell about its proliferation must represent a consensus decision shared with the cells that reside in its neighborhood.
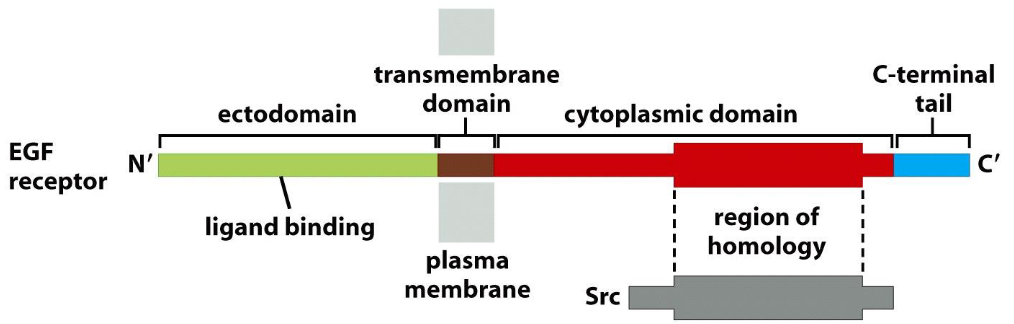
The epidermal growth factor receptor (EGF-R) consists of three functional domains, in which part of the cytoplasmic domain shows homology with Src:
Ectodomain: 621 AA
Transmembrane domain: 23 AA
Cytoplasmic domain: 542 AA
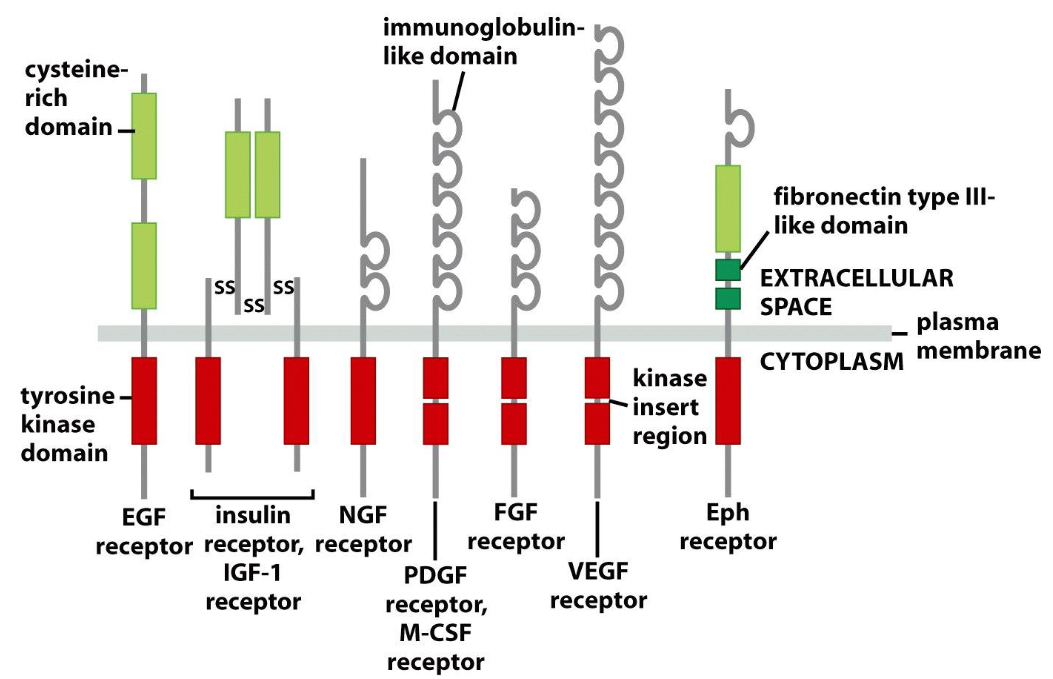
Structure of tyrosine kinase receptors (RTKs) have three functional domains:
Ectodomain: cysteine rich domain/ immunoglobin-like domain
Transmembrane domain: inside plasma membrane
Cytoplasmic domain: tyrosine kinase domain
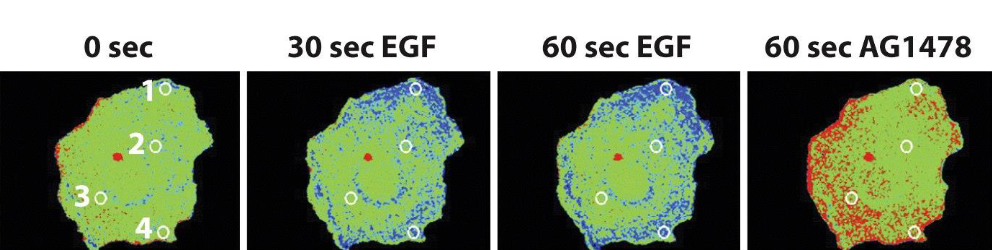
Formation of phospho-tyrosine on the EGF-R following ligand addition appears in:
➢ Monkey kidney cells.
➢ In blue: receptor phosphorylation above the basal level; In red: receptor phosphorylation below the basal level.
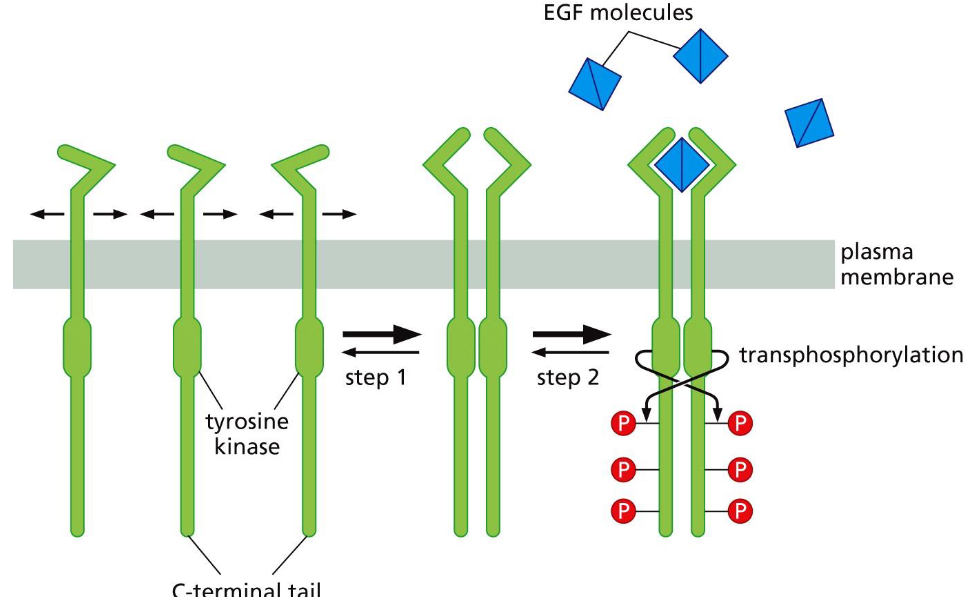
Two critical changes of tyrosine kinase Receptors (RTKs) following ligand binding:
➢ Dimerization.
➢ Phosphorylation (transphosphorylation) of the cytoplasmic domain
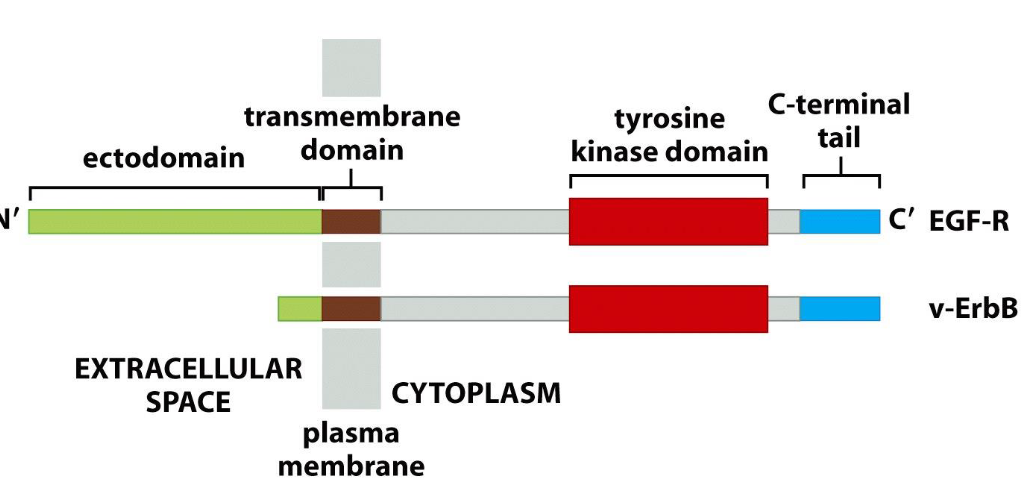
Deletion of the ectodomain of EGF-R results in:
activation of the receptor.
[v-ErbB : an oncoprotein in avian erythroblastosis virus (AEV) ]
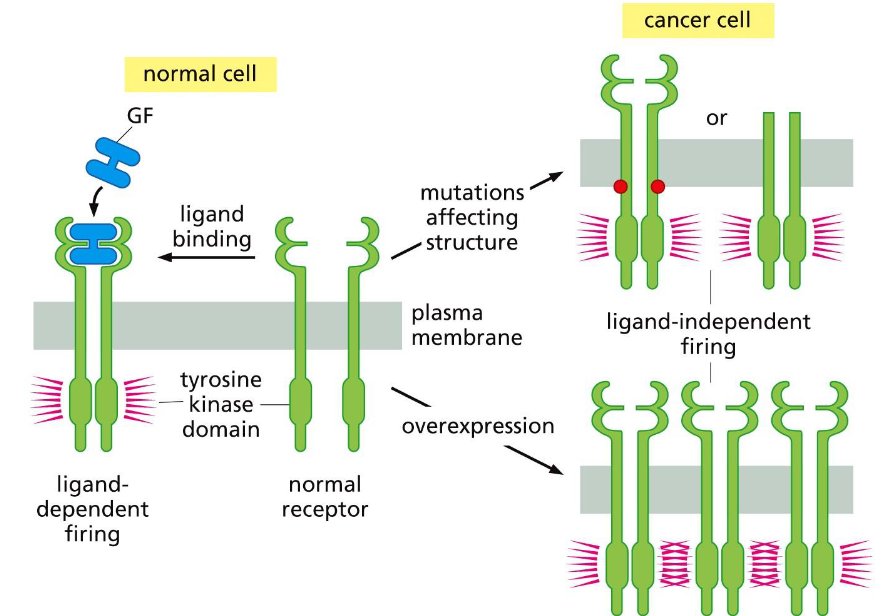
Deregulation of receptor firing happens through:
Receptor mutation and overexpression.
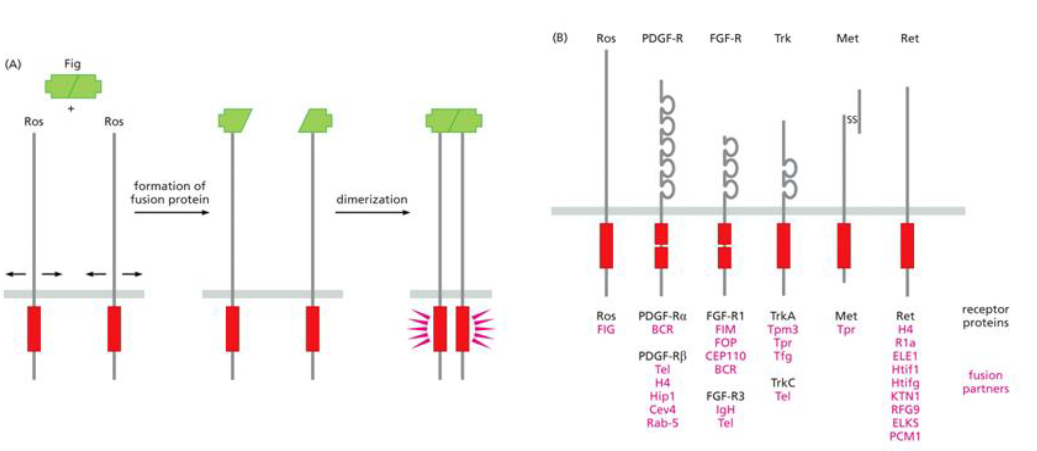
Gene fusion causing constitutively dimerized receptors involves the
ROS receptor tyrosine kinase and the FIG (Fused in Glioblastoma) gene fusing to form a dimer.
Deregulation of receptor firing, autocrine signaling:
A form of signaling in which a cell manufactures its own mitogens.
Deregulated activation caused by abnormal dimerization of RTKs summary:
-Overexpression of receptors: dimerization via collision of RTKs.
- Mutation (amino acid substitutions) of RTKs.
- Truncation of the ectodomain of RTKs.
- Fusion of RTKs to other proteins that dimerize.
How many proteins have general structures of the EGF-R and PDGF-R in human genome
59 tyrosine kinase receptors (RTKs)
Other receptors in human cells
➢ 59 tyrosine kinase receptors (RTKs) (e.g., EGF-R).
➢ 24 Integrin receptors.
➢ 11 Transforming growth factor-β (TGF-β) receptors.
➢ 4 Notch receptors.
➢ 2 Patched receptors.
➢ 10 Frizzled receptors.
➢ More than 1000 G-protein-coupled receptors (GPCRs).
➢ 48 nuclear receptors.
Why is autocrine signaling an intrinsically destabilizing force for a normal tissue?
Breaks cell–cell interdependence
Introduces self-sustaining growth loops
Undermines collective control mechanisms
gives single cells a competitive growth/survival advantage
Signaling cascades:
ordered sequences of biochemical reactions inside the cell with high specificity
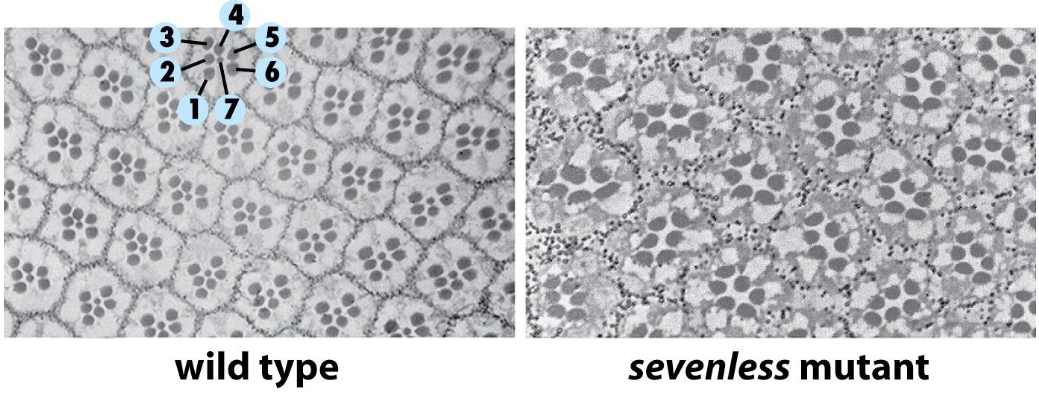
Structure of the fruit fly eye - the ommatidia
➢ Each ommatidium is formed from a series of seven cells.
➢ Sevenless gene encodes a homolog of the FGF-R (an RTK).
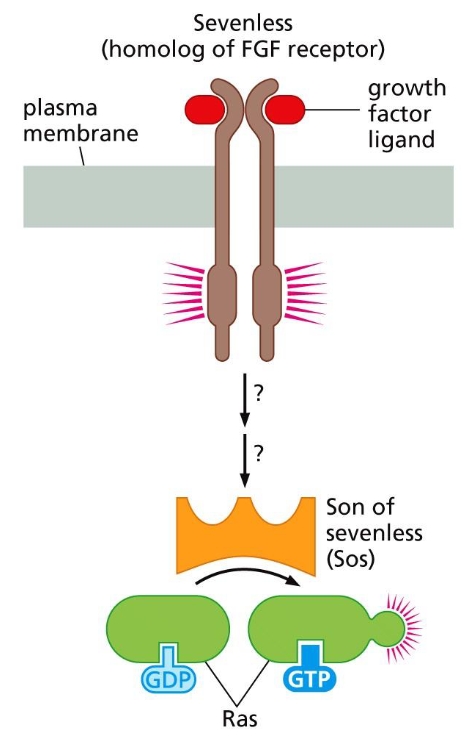
Sos is the ___ stimulator of Ras:
Upstream stimulator(downstream signaling)). It is a guanine nucleotide exchange factor (GEF) that activates Ras by catalyzing the exchange of GDP for GTP.
Sos promotes GDP → GTP exchange on Ras, converting Ras into its active, GTP-bound state. cascade: RTK→ Grb2→ Sos → Ras or RTK→ Shc→ Grb2→ Sos→ Ras.
Localization model:
RTKs affect the physical location of downstream components without necessarily changing their intrinsic activities.
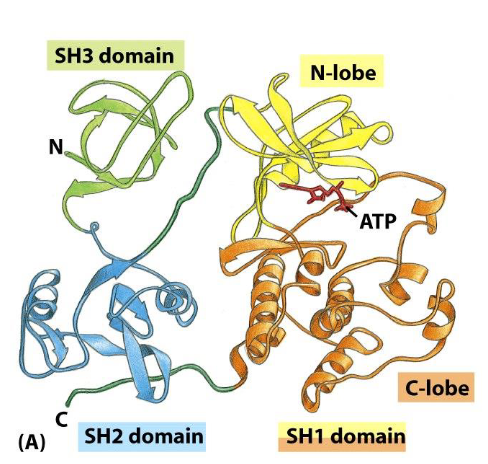
The Src protein contains three domains:
SH1 (catalytic domain), SH2 (binding to pY- containing peptide), and SH3 (binding to proline-rich sequence domain)
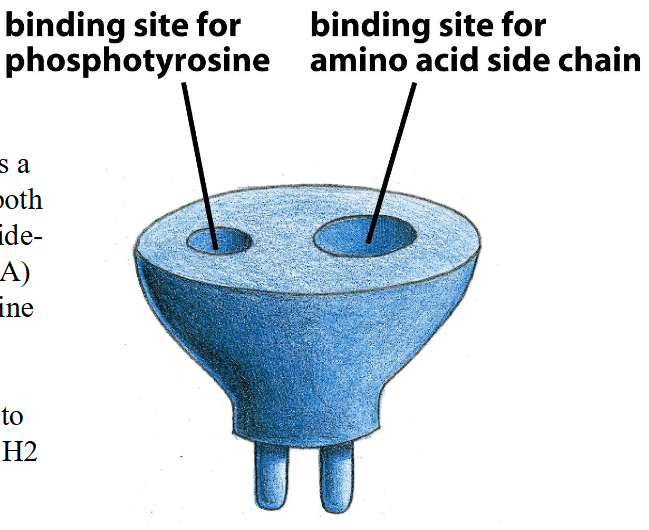
Structure and function of SH2 groups
➢ The SH2 domain functions as a modular plug: it recognizes both a phospho-tyrosine and the side- chains of amino acids (3-6 AA) that flank this phospho-tyrosine on its C-terminal side
➢ A typical SH2 domain is composed of 100 amino acid residues. Assembled from a pair of anti-parallel β pleated sheets surrounded by a pair of α helices.
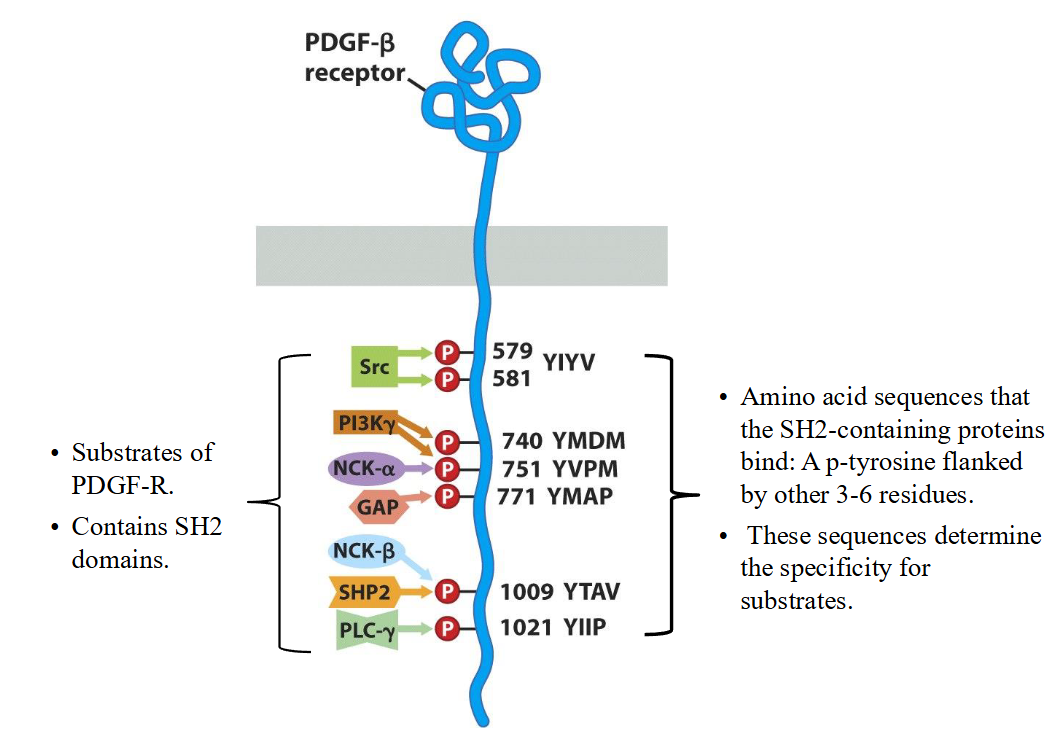
Explain attraction of signal-transduction proteins by phosphorylated receptors
Phosphorylated receptors act like “docking stations.” They attract signaling proteins through phosphotyrosine-recognizing domains, localize them to the membrane, and allow precise and coordinated activation of downstream signaling pathways.
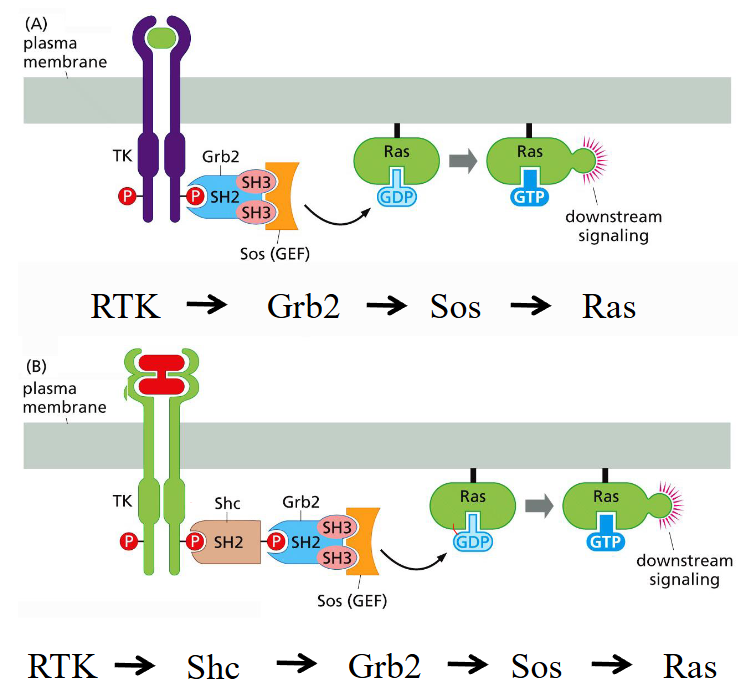
Why are intermolecular links forged by the Grb2 and Shc bridging proteins important?
It provides modularity: Grb2 and Shc can connect many different receptors to the same Ras pathway.
It enforces conditionality: Sos is only recruited when the receptor is phosphorylated, preventing accidental Ras activation.
It allows signal amplification and integration: multiple Grb2/Sos complexes can dock, producing a strong and tunable signal.
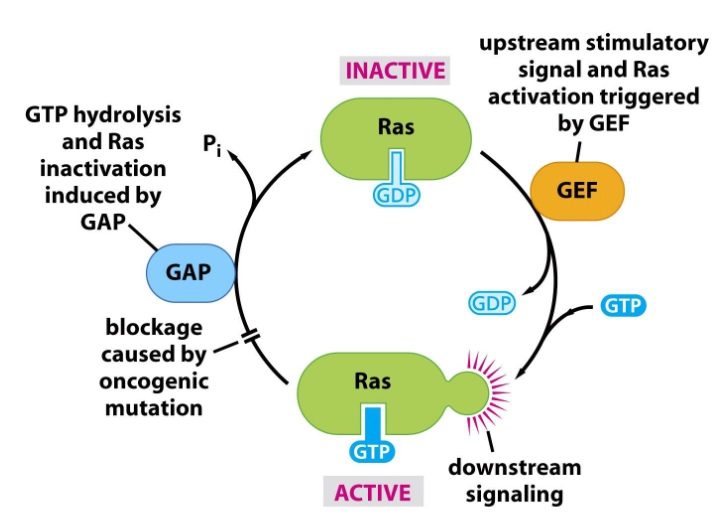
Ras signaling cycle determined by
-GTP (active) /GDP (inactive) binds to Ras.
-GDP to GTP by GEF: guanine nucleotide exchange factor.
-GAP: GTPase-activating proteins. GTPase: hydrolyzes GTP to GDP.
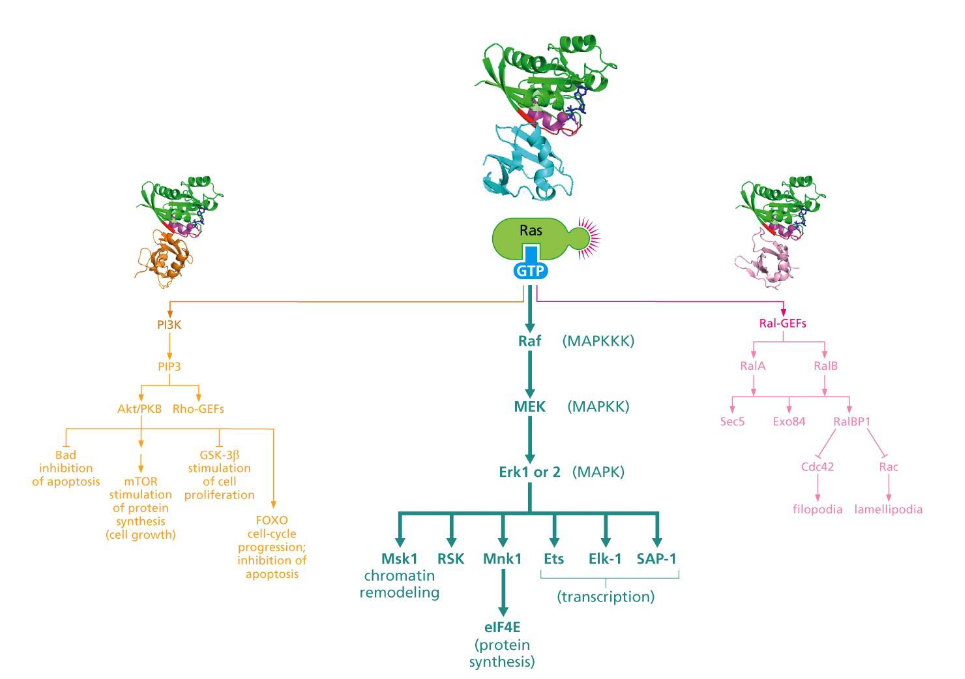
Three important signaling pathways downstream of Ras
-MAPK pathway, PI3K pathway, Ral-GEFs pathway
-Signaling pathways are inter-connected
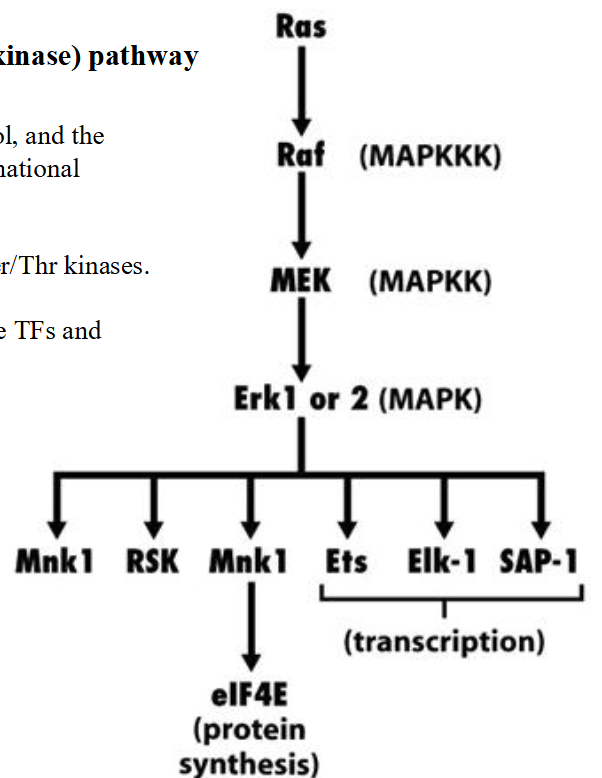
MAPK (mitogen-activated protein kinase) pathway
➢ Activated Ras attracts Raf from cytosol, and the binding of Raf to Ras leads to conformational changes of Raf.
➢ MEK: phosphorylates both Tyr and Ser/Thr kinases.
➢ Erk 1 and 2 phosphorylate and activate TFs and other proteins.
- Deregulation of MAPK pathway contributes to certain cancer phenotypes (loss of contact inhibition, anchorage independence, etc).
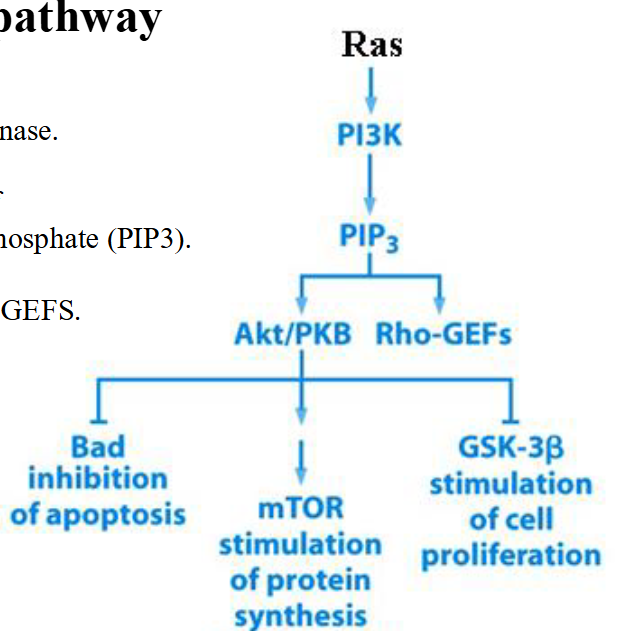
The PI3 kinase pathway
➢PI3K: Phosphatidylinositol 3-kinase.
➢Function of PI3K: Synthesis of phosphatidylinositol (3,4,5) triphosphate (PIP3).
➢PIP3 attracts Akt/PKB and Rho-GEFS.
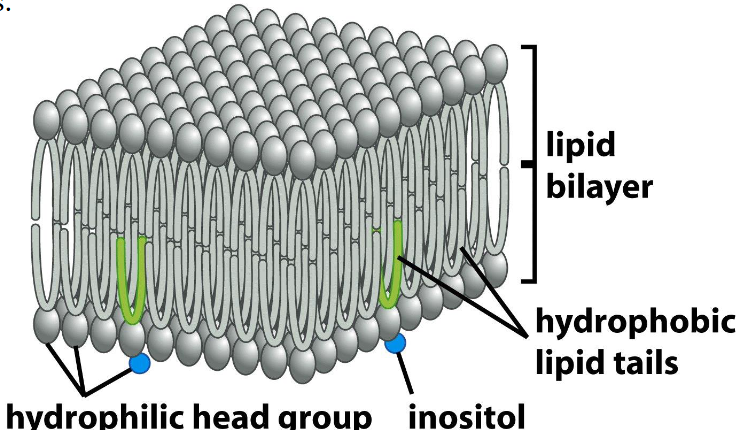
Lipid bilayers
➢ Phospholipid is composed of the hydrophilic head groups and the hydrophobic tails.
➢ A small minority of the head groups contain inositol sugars.
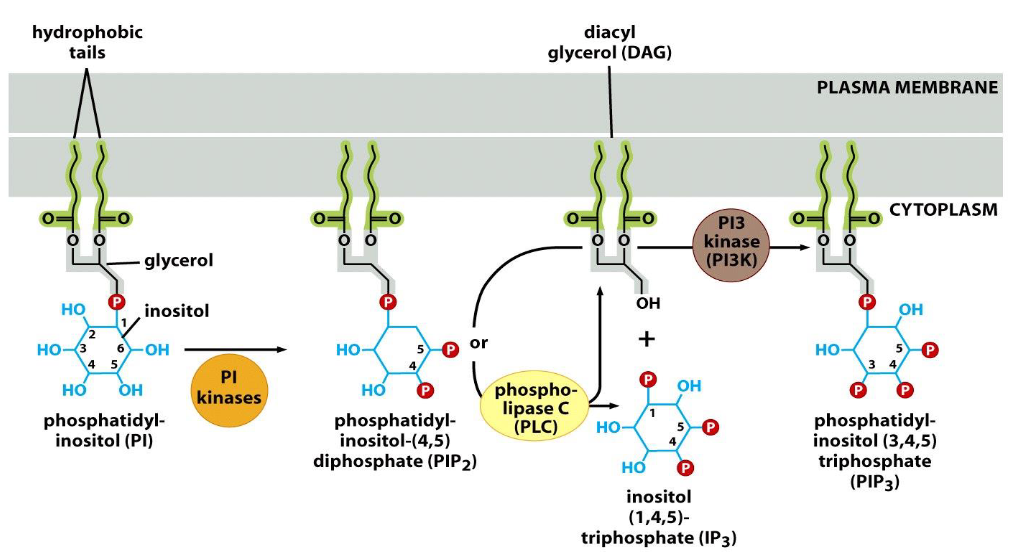
Phosphatidylinositol (PI), and its phosphorylation
➢ PI is composed of 3 parts: 1) two fatty acids with long hydrocarbon tails, 2) glycerol, and 3) phosphate & inositol.
➢ PI, PI (4,5)-diphosphate (PIP2), and Pl (3,4,5)-triphosphate (PIP3) are tethered to plasma membrane.
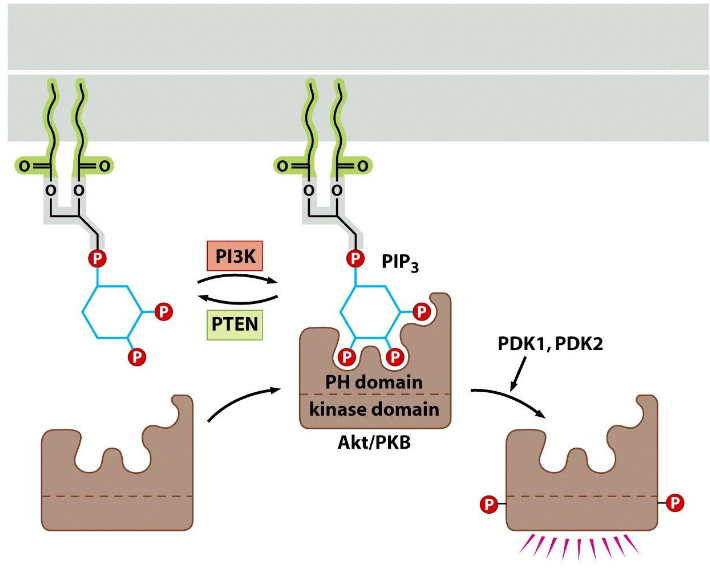
Regulation of PIP3 synthesis and the activation of Akt/PKB
➢ PIP3 formation is controlled by PI3K and PTEN.
➢ PIP3 serves as a docking site for Akt/PKB.
➢ Once binds to PIP3, Akt/PKB becomes phosphorylated and activated.
Tumor suppressor genes:
The anti-growth genes.
➢ These genes are as important as oncogenes.
Retinoblastoma
➢1 in 20,000 children.
➢ Diagnosed from birth up to 6-8 year old.
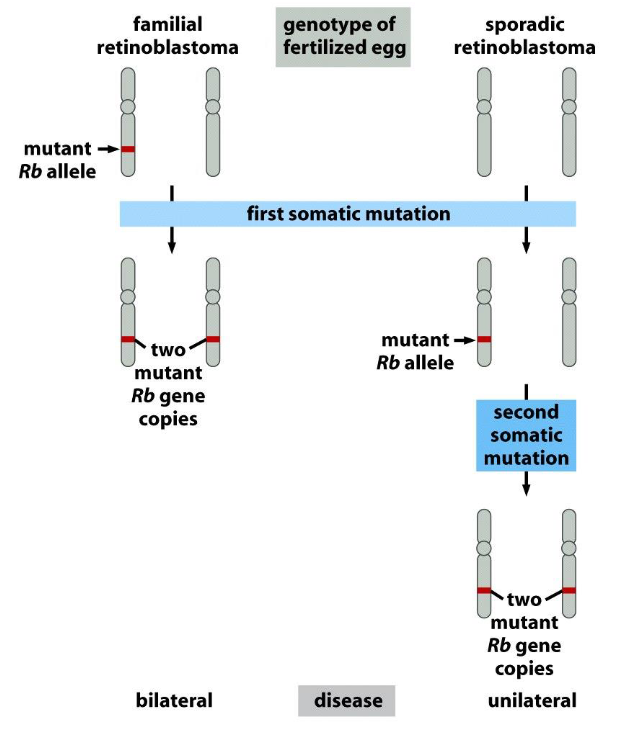
Unilateral versus bilateral retinoblastoma
➢ Unilateral retinoblastomas: Affect only a single eye; the sporadic form.
➢ Bilateral retinoblastomas: Affect both eyes; the familial form.
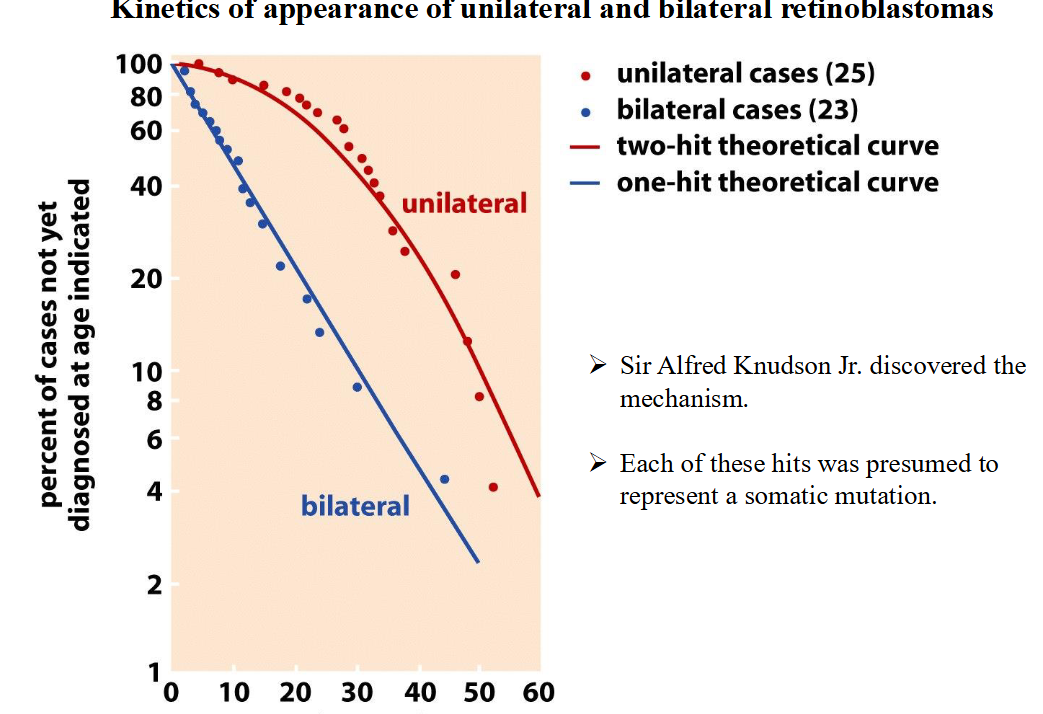
Dynamics of retinoblastoma formation
➢ Bilateral retinoblastomas require one hit for tumor formation.
➢ Unilateral retinoblastomas require two hits for tumor formation.
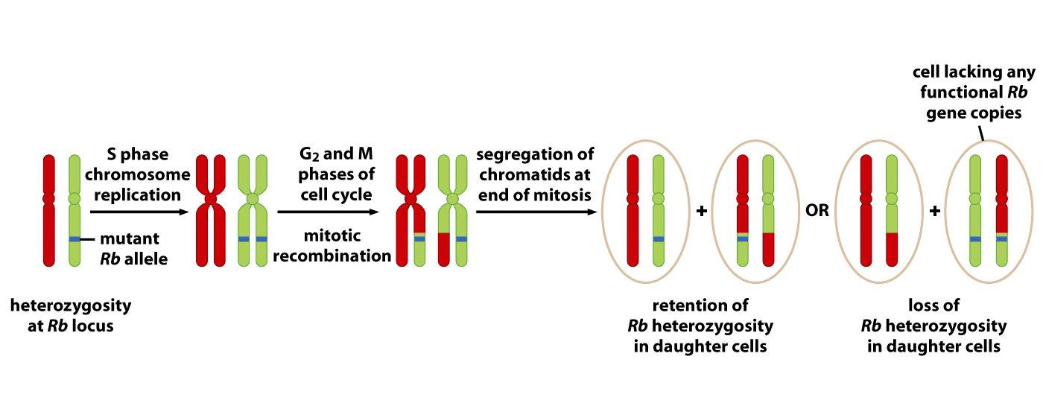
Mitotic recombination and loss of heterozygosity (LOH)
➢ Mitotic recombination: recombination occur during cell proliferation.
➢ Loss of heterozygosity (LOH) : also called “allelic deletion”; a genetic alteration that
converts a chromosome region from heterozygous to homozygous.
-Gene conversion and Chromosomal nondisjunction also contribute to LOH
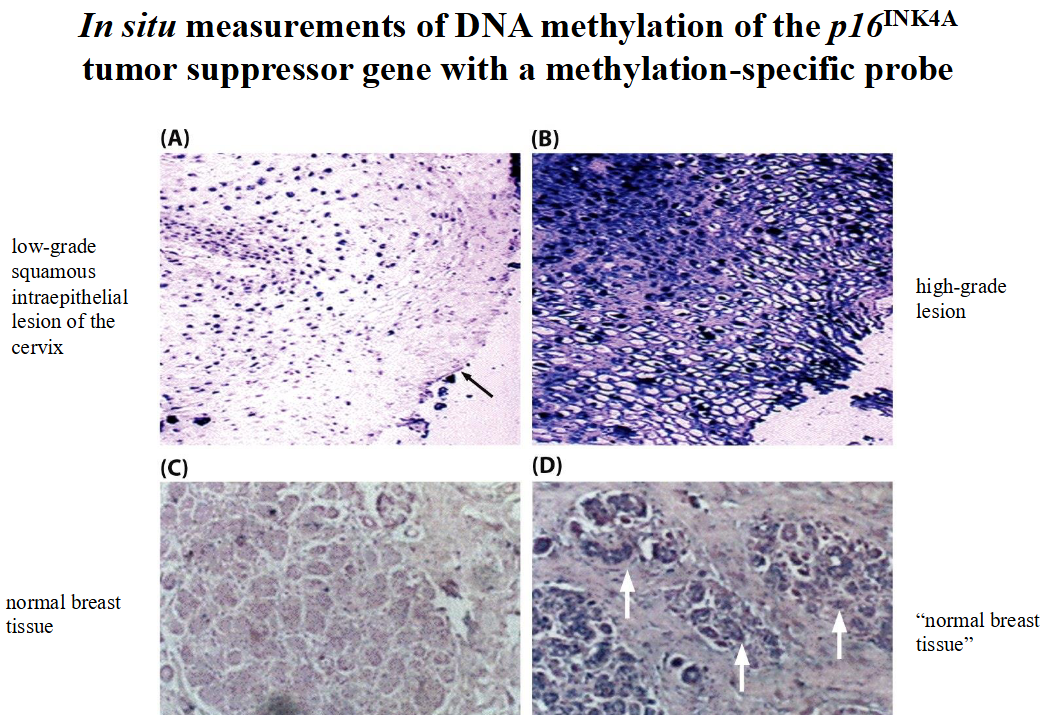
DNA methylation, an important mechanism in shutting down genes, does what:
➢ covalently attaches a methyl group to a cytosine base.
➢ In mammalian cells, the methylation is found only when cytosines are located in a position that is 5’ to guanosines: MeCpG.
➢ When MeCpG occurs in the vicinity of a gene promoter, the expression of the gene can be repressed.
DNA methylation and LOH work together to shut down suppressor genes
➢ One copy is methylated, and the second is lost through LOH.
➢ Frequency: LOH > methylation > sporadic mutation
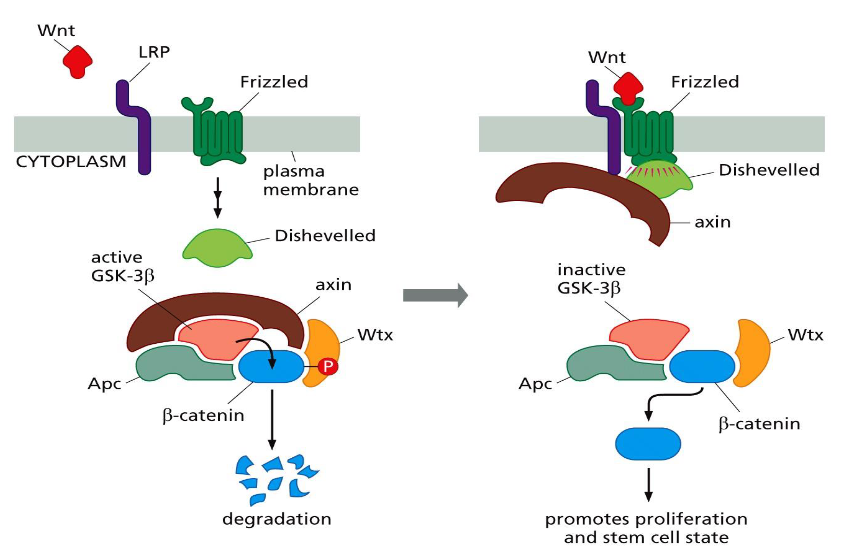
Wnt-β-catenin pathway
➢ In the absence of Wnt, glycogen synthase kinase-3β (GSK-3β) phosphorylates β catenin, leading to degradation of β-catenin.
➢ Binding of Wnt to the Frizzled receptors causes inhibition of GSK-3β via Dishevelled and axin, preventing phosphorylation and degradation of β-catenin.
β-catenin associates with TF Tcf/Lef in the nucleus and does what?
Drives cell proliferation.
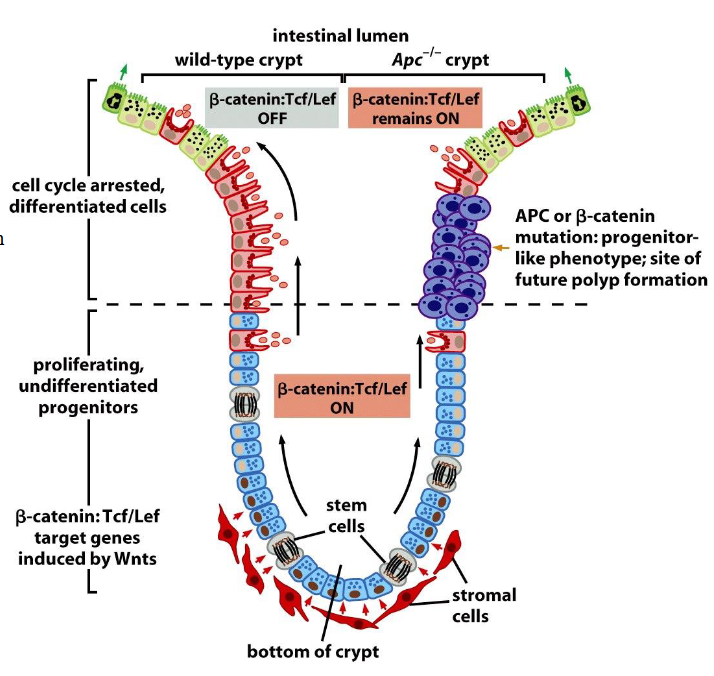
β-catenin and the biology of colonic crypts in normal vs tumor cells
Normal cells:
➢ Stem cells receive Wnt signal from stroma. β-Catenin interacts with Tcf/Lef, and promotes proliferation in stem cells. When cells move upward, stimulation by Wnts decreases, leading to increased degradation of β-catenin. Cells enter apoptosis after 3-4 days.
Tumor cells:
➢ When the Apc protein is defective, β-catenin levels remain high even in the absence of intense Wnt signaling. Cells stop migrating upward, accumulate within crypts, and ultimately generate an adenomatous polyp.
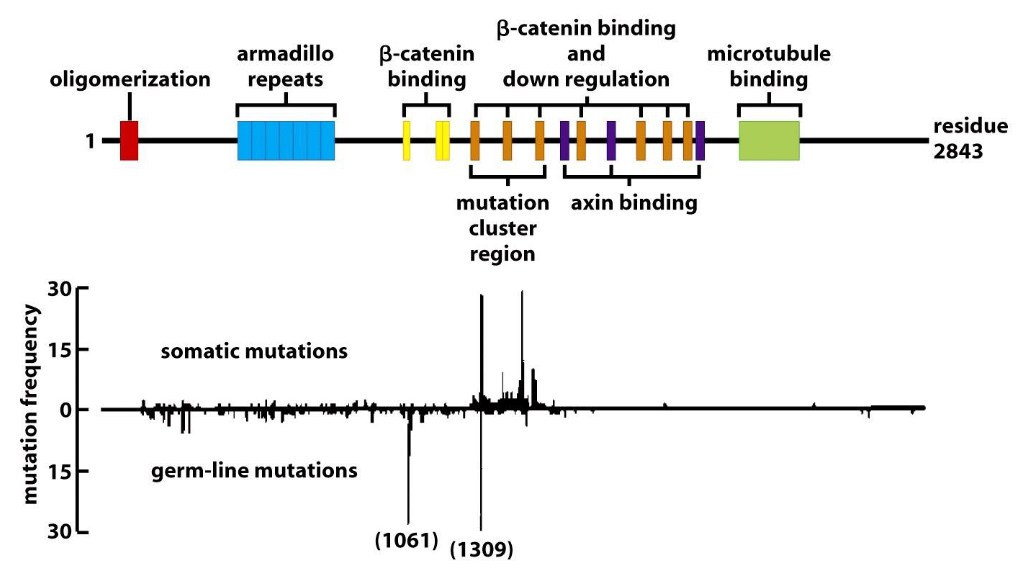
Apc protein contains multiple protein binding domains, and the gene encoding Apc___:
is frequently mutated.
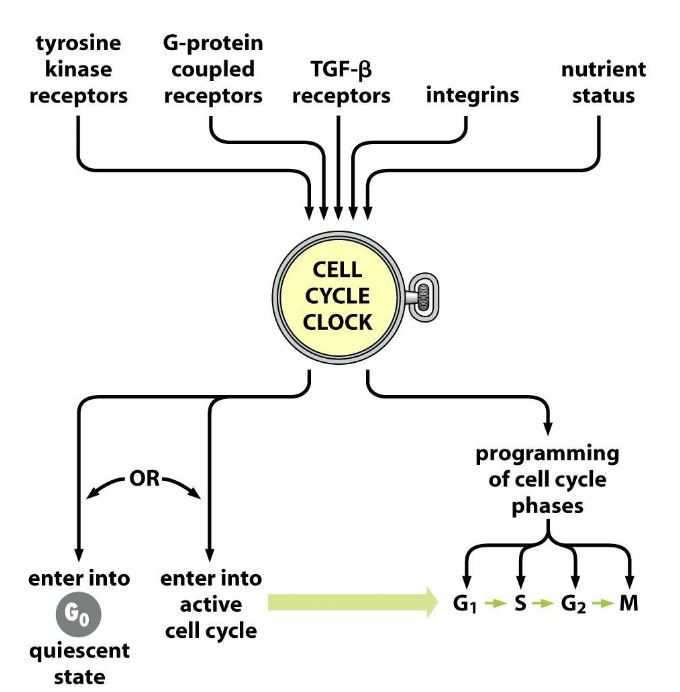
Cell cycle clock is the central governor of:
growth and proliferation.
Cell cycle clock:
-located in the nucleus.
- a network of interacting proteins (a signal processing circuit) that receives signals from various sources both outside and inside the cells, integrates them, and decides the cell’s fate.
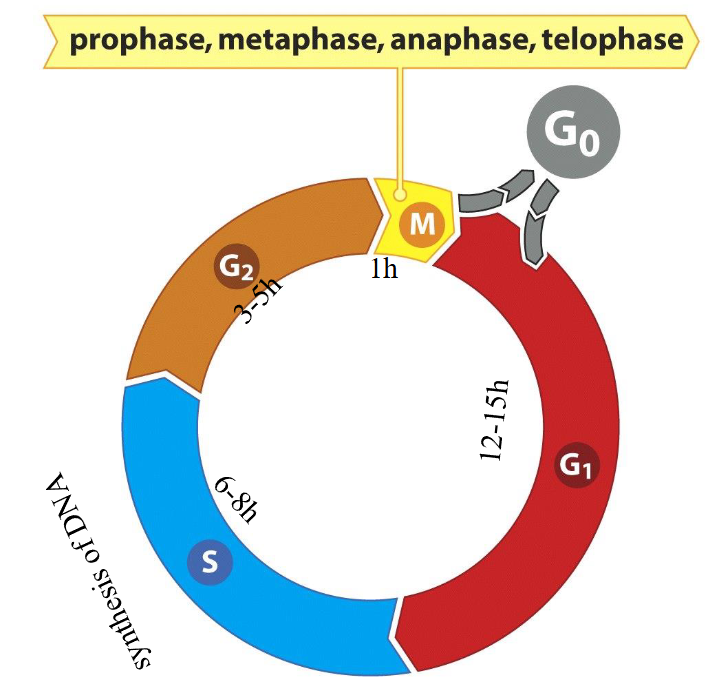
The mammalian cell cycle is divided into four phases:
G1, S, G2, M phases
M phase divided into Prophase, Metaphase, Anaphase, Telophase
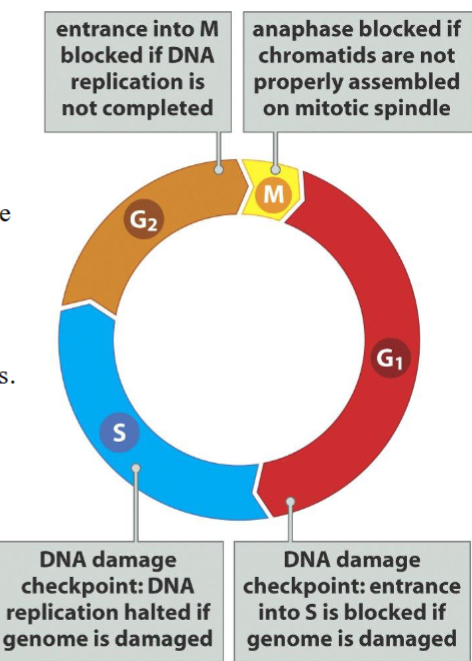
Checkpoints (checkpoint control):
are quality control mechanisms that ensure the fidelity of cell division in eukaryotic cells.
➢ Cells are allowed to advance into the next phase only if all the requisites are met
Responsiveness to extracellular signals during the cell cycle
➢ Cells respond to extracellular mitogens and inhibitory factors (such as TGF-β) only in a discrete window of time that begins at the onset of G1 and ends just before the end of G1.
➢ Restriction (R) point:
The point in time when the cell must make the commitment to advance through the remainder of the cell cycle through M phase, to remain in G1, or to retreat from the active cell cycle into G0.
➢ After this point→ S→ G2→ M progression is the same in normal and caner cells.
Cyclin:
are the regulatory subunits of the heterodimeric protein kinases that control cell cycle events
CDKs:
a group of serine/threonine kinases that are involved in the regulation of the cell cycle
Cyclin-CDKs complexes
-engine of the cell cycle clock
-Combining of cyclin A to CDK2 increase the catalytic activity of CDK2 by 400,000-fold.
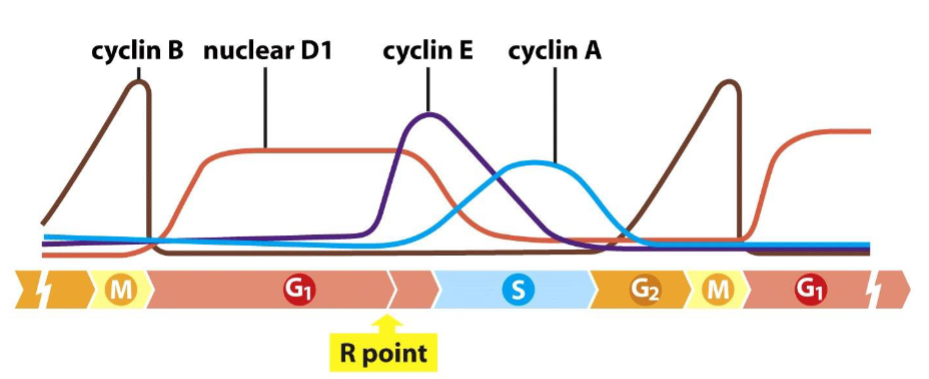
Fluctuation of cyclin levels during the cell cycle in most mammalian cells are due to:
➢ Extracellular signals (especially mitogenic growth factors) strongly influence the levels of D-type cyclins.
➢ The fluctuations of cyclins B, E, and A are tightly coordinated with the schedule of advances through the various cell cycle phases (pre-programmed).
Various pathways are involved in the control of cyclin D1 expression
➢ D-type cyclins serve to convey signals from extracellular environment to the cell cycle clock in the cell nucleus.
CDK inhibitors (CDKIs):
A group of proteins that affect the activities of cyclin-CDK complexes.
There are seven important:
─ Four INK4 (inhibitors of CDK4); affect only CDK4/CDK6
─ Three others affect CDK2 and CDC2.
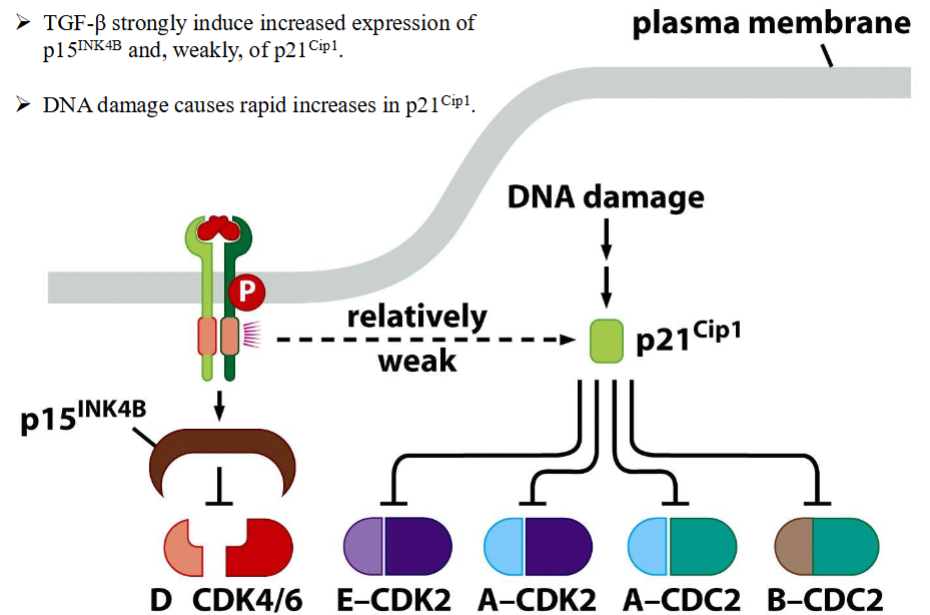
Control of cell cycle progression by TGF-β and DNA damage
➢ TGF-β strongly induce increased expression of p15-INK4B and, weakly, of p21-Cip1.
➢ DNA damage causes rapid increases in p21-Cip1.
pRb is:
-the molecular governor of the R point transition.
-Control of this protein function is perturbed in most, if not all, human cancers
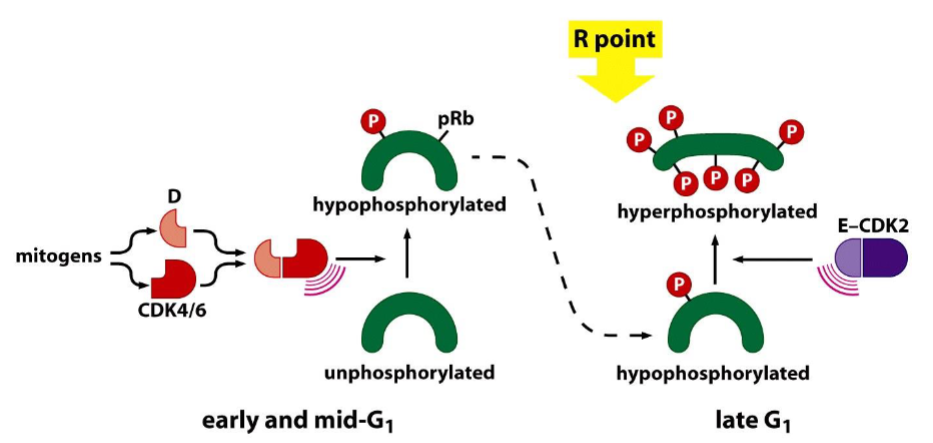
Control of the restriction-point transition by mitogens:
➢ The levels of D-type cyclins are controlled largely by extracellular signals.
➢ D-CDK4/6 complexes drive pRb hypophosphorylation (active form of pRb).
➢ Levels of cyclin E increase dramatically at the R point.
➢ E-CDK2 complexes drive pRb hyperphosphorylation (inactive form).
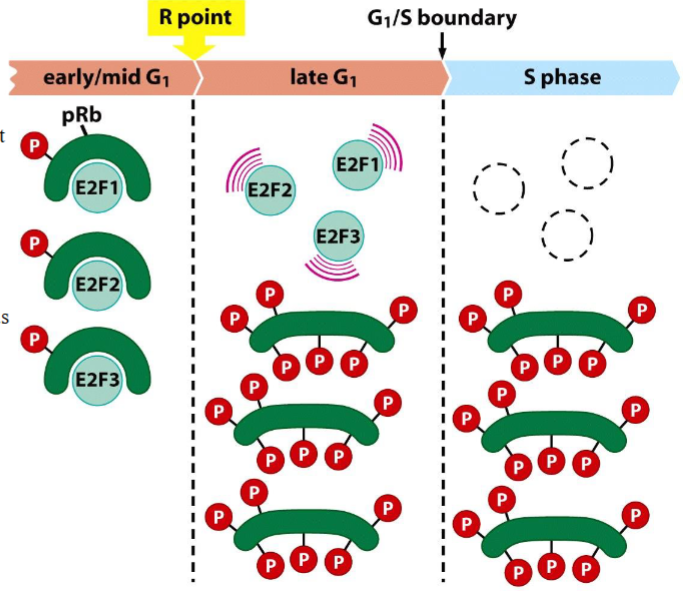
pRb controls the transcription activity of E2Fs
➢ When pRb binds to E2Fs, it blocks their transcription-activating domain.
➢ After being hyperphosphorylated at the R point, pRb release E2Fs, allowing E2Fs to function as TFs.
➢ As cells enter into S phase the E2Fs are inactivated and/or degraded.
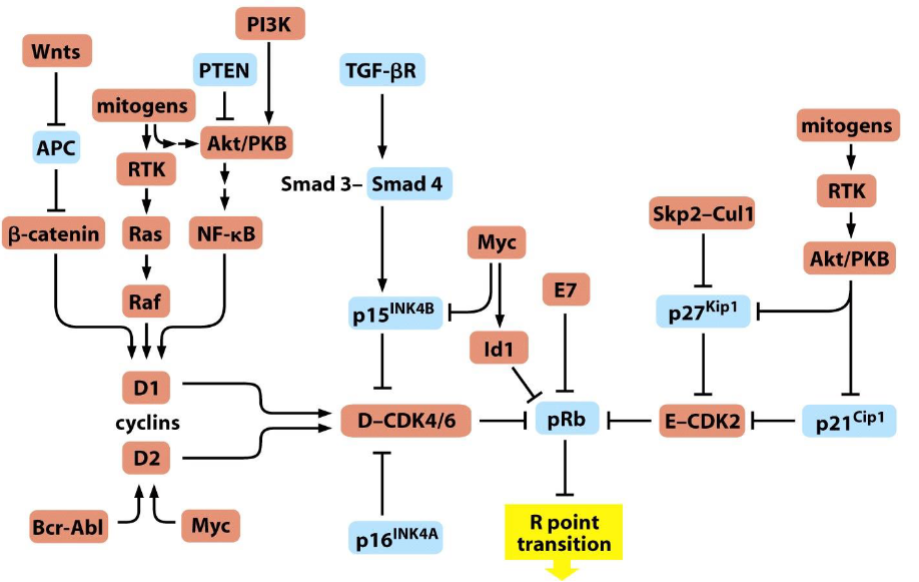
Perturbation of the R-point transition in human tumors
➢ Elements that favor advance through the R point are drawn in red, while those that undertake to block this advance are shown in blue
Concerning growth factors, what mechanisms might be employed by the cell to reduce its responsiveness to a growth factor?
Receptor inactivation, internalization, and degradation
Ligand depletion or sequestration
Negative feedback loops and inhibitory proteins
Changes in gene expression reducing downstream sensitivity
Growth factor receptor firing following ligand binding is often dependent on what process?
Growth factor receptor activation depends on ligand-induced receptor dimerization, not just a conformational change in a monomer. (or molecular change in the receptor)
In what ways are the heterotrimeric G-proteins similar to the low-molecular-weight G-proteins (like Ras), and in what way do they differ fundamentally?
Both act as GTP-driven molecular switches.
Heterotrimeric G-proteins are multi-subunit complexes controlled by GPCRs.
Low–molecular–weight GTPases (like Ras) are single proteins regulated by distinct GEFs and GAPs downstream of RTKs and other receptors.
In what ways has the study of lower organisms like yeast, flies, and worms, been useful in characterizing the intracellular signaling cascades and the receptors operating at the cell surface?
Showed how receptors and intracellular cascades control development, growth, and homeostasis.
Provided direct insight into human disease, especially cancer. Genetically tractable: allowed forward genetics to dissect pathways. Uncovered highly conserved signaling cascades (GPCRs, RTKs, Ras, MAPK, Wnt, Hedgehog, TGF-β).
Integrins exhibit a novel type of control, termed "inside-out signaling," in which intracellular signals dictate the affinity of these receptors for their various extracellular ligands. Why is such signaling essential to the process of cell motility.
Inside-out signaling lets cells tune integrin affinity on demand, enabling the adhesion–traction–release cycle of motility. Without it, cells could not migrate effectively.
What mechanisms ensure cell signaling specificity in a signaling cascade rather than being broadcast non-specifically to "unintended" targets in the cytoplasm?
Scaffolding kinases into dedicated complexes.
Localizing signals to defined compartments (plasma membrane, endosomes, nucleus).
Using modular binding domains as precise docking codes.
Employing temporal feedback controls to terminate signals quickly.
Restricting activation to properly assembled complexes.
Insulating parallel pathways that use overlapping components.
What are some distinct molecular mechanisms that lead to the conversion of a proto-oncogene into in active oncogene?
Point mutation
Gene amplification
Chromosomal translocation → regulatory control change
Chromosomal translocation → fusion oncogene
Insertional mutagenesis by viruses
Epigenetic Deregulation
Post-Translational Deregulation (β-catenin)
What factors determine the lifetime of the activated state of a Ras oncoprotein?
The Intrinsic GTP hydrolysis rate (slowed by oncogenic mutations). GAP activity (accelerates shutoff), GEF activity (promotes reactivation). GTP/GDP availability (favors GTP binding).
Localization and modifications (control access to regulators). Feedback loops (fine-tune signaling duration).
Why is the inheritance of mutant, activated oncogenes responsible for only a small proportion of familial cancer syndromes, whereas the inheritance of defective tumor suppressor genes is responsible for the lion's share of these diseases?
Inheritance of mutant activated oncogenes (gain of function) are disruptive/lethal to embryonic development.
In contrast, inheritance of one defective tumor suppressor allele (loss of function) is tolerated, until second allele is lost; (Knudson’s “two-hit” model)
What factors may determine why inactivation of TSG may occur at a higher frequency than the activation of an oncogene?
Inactivation = any nonsense mutation, truncation, or deletion. Activation requires precise structural change.
The mutational “target size” for TSG inactivation is much larger than that for oncogene activation. One hit can be a large chromosomal deletion, eliminating both copies in one event (LOH). When one TSG suppressor is lost, others are lost more easily.
How are the outcomes of the loss of TSG function and acquisition of an active oncogene similar at cell-biological level?
What are the differences?
At the cell-biological level, this leads to functionally identical outcomes: Sustained mitogenic signaling; Unrestrained cell cycle entry; Resistance to apoptosis; Enhanced motility/invasion
Mechanistic differences: Oncogenes = gain-of-function mutations (turning signals on). TSGs = loss-of-function mutations (removing brakes).
Why do some TSGs undergo LOH in fewer than 20% of tumors of a given type.
Why do such low rates of LOH complicate the identification and molecular isolation of such genes?
Some TSGs show low rates of LOH because they are inactivated by alternative mechanisms (point mutations, methylation, pathway redundancy)
If only ~20% of tumors show LOH at a given locus, the statistical signal is weak. It doesn’t stand out compared to background chromosomal instability
What criteria needs to be satisfied in categorizing a gene as a TSG?
Inactivation in tumors (mutations, deletions, methylation).
Biallelic loss consistent with the two-hit hypothesis.
Functional assays showing loss promotes tumor traits, while restoration suppresses them.
Genetics linking germline defects to cancer predisposition.
Biological role consistent with growth restraint, genome stability, or apoptosis.
What factors might influence the identities of the tissues affected by an inherited, defective allele of a TSG?
Pathway dependence of that tissue (is the TSG critical there?).
Cell proliferation rates (fast-cycling tissues are vulnerable; APC/Wnt).
Environmental exposures (UV, tobacco, diet).
Backup mechanisms (redundancy vs. dependence).
Hormonal/developmental context (estrogen, thyroid signals, PTEN.).
Stem cell dynamics (mutation retention vs. dilution/ Colon crypt stem cells)
What evidence might be produced to demonstrate that a group of a candidate TSGs function in the same regulatory pathway within normal cells?
TSGs need to be in the same regulatory pathways to regulate metabolic processes. Some methods include:
Genetic epistasis, Mutual exclusivity in tumors (one mutation is sufficient, no need for both), Functional rescue (upstream/downstream compensation) Physical interactions, Shared downstream phenotypes, Co-expression/regulation
Why is pRb function compromised in human tumors through mutations of its encoding gene, whereas p107 and p130, have virtually never been found to suffer mutations in the genomes of cancer cells?
RB1 is mutated in cancers because it is the dominant, non-redundant enforcer of the G1/S checkpoint.
p107 and p130 play supporting roles that can be bypassed by oncogenic signaling or viral proteins. Mainly regulate quiescence (G0), have redundant roles, can be inactivated indirectly,
Why have DNA tumor viruses evolved the ability to inactivate pRb function?
pRb is the key gatekeeper of the G1→S transition
doing so unlocks the host’s S-phase machinery, providing the DNA synthesis enzymes and substrates the virus needs to replicate its genome.
How might loss of a CDK inhibitor’s function affect the control of cell cycle advance?
Loss of a CDK inhibitor removes a key brake on cyclin-CDK activity, leading to uncontrolled phosphorylation of pRb, inappropriate activation of E2Fs, premature S-phase entry, and loss of checkpoint control, driving uncontrolled proliferation and increasing mutation accumulation.
What molecular mechanisms ensure that once the decision to advance through the restriction point has been made, an irreversible commitment is made to complete the remaining phases of the cell cycle through M phase?
Hyperphosphorylation of pRb, leads to full E2F release, triggering S-phase gene expression. The cascade is sequential and coupled, so retreating back to G1 is blocked.
Checkpoints operate to halt further progression if damage is detected, but they generally cannot “reset” the G1 commitment once the replication machinery is engaged.
How are the decisions of cell growth versus quiescence coupled mechanistically with the decisions governing cell differentiation? Why must these two processes be tightly coupled?
Cell cycle activation vs. quiescence(inactivity) is governed by the Rb–E2F checkpoint. Quiescence ensures that energy and resources are dedicated to function, not growth.
Tight coupling is essential to: prevent cancerous growth, allow proper tissue specialization, maintain stem cell pools, and protect differentiated cells from replication stress.
How do cells ensure that the transcription-activating functions of the E2F transcription factors are limited to a narrow window of time in the cell cycle?
What might occur if E2F function were allowed to continue throughout the cell cycle?
Cells limit E2F activity to a short window at the G1→S transition by combining: hypophosphorylated pRb binding and repressing E2F, proteasomal degradation of E2Fs, feedback from cyclin A/CDK2
If E2F activity persisted, cells would risk uncontrolled S-phase gene expression, Loss of cell-cycle order, apoptosis, or cancer risk.
In what ways does the Myc oncoprotein deregulate cell proliferation and differentiation?
Proliferation: Myc drives cyclins/CDKs, represses inhibitors, activates E2Fs, and fuels biosynthesis.
Differentiation: Myc blocks lineage-specific transcription factors, prevents cell-cycle exit, and maintains an undifferentiated chromatin state.
Together, these effects lock cells into a proliferative, undifferentiated state, creating a fertile ground for malignant transformation.
Why is it important that the cell cycle clock never runs backward?
DNA replication licensing is a one-time event, Checkpoint satisfaction depends on completion of the previous step. Biological consequences if reversal occurred:
Re-replication of DNA → aneuploidy, mutations, oncogenesis. Chromosome loss. accumulation of DNA damage. Tissue dysfunction/cancer
What evidence suggest that the cell cycle clock may be organized differently in certain cell types or stages of embryonic development
Experiments in embryos, knockout animals, cell-free systems, and stem vs differentiated cells all suggest that the cell cycle is not a fixed program but instead reorganized depending on developmental stage or cell type.
Early embryos: rapid S–M cycles, no checkpoints.
Later tissues: canonical G1–S–G2–M with checkpoints and growth control.
Differentiated cells: exit into G0 and couple cycle control tightly to differentiation.