(pt 2) exam #2 - immunohematology (cls 544)
1/65
Earn XP
Description and Tags
HDFN + detection of antibodies
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
66 Terms
hemolytic disease of the fetus and newborn (HDFN)
destruction of the RBCs of a fetus or neonate by maternal antibodies that attached to corresponding antigens on baby's RBCs
Antibodies transferred through the placenta stimulated naturally (ABO) or by previous pregnancy or transfusion during current pregnancy
Results in anemia, accumulation of bilirubin, enlarged spleen and liver
facts of HDFN
Pathogenesis of HDFN elucidated discovery of the Rh antigen
Discovery of Rh-immune globulin in 1968 led to prevention of HDFN caused by anti-D
etiology of HDFN
Sensitization of blood group antigens occurs with previous pregnancies or RBC transfusions
Fetal RBCs possessing a paternal antigen foreign to mom can enter the maternal circulation causing feto-maternal hemorrhage (FMH)
FMH more likely to occur at delivery once placenta separates from the uterine wall
pathophysiology of HDFN
Associated causes of HDFN include ABO, Rh or other blood group incompatibility between mother and infant
Four stages of pathogenesis
Exposure of mother to fetal RBC antigens with subsequent production of maternal blood group antibodies
Placental transfer of the maternal antibodies to the fetus w attachment to fetal RBC antigens
Subsequent immune destruction of the antibody-coated fetal RBCs
Clinical manifestations resulting from destruction of the "marked" RBCs
disease progression of HDFN in fetus
RBCs are destroyed → fetus becomes anemic
Erythroblastosis fetalis--immature nucleated RBCs released into fetal circulation from BM
response to anemia → RBCs cells made in liver and spleen (in utero)
Hepatosplenomegaly occurs
Progressive anemia causes tissue hypoxia → increased cardiac output
Heart beats rapidly with eventual heart failure
Hydrops fetalis--significant fluid buildup characterized by respiratory / circulatory distress
At birth, affected newborns are anemic but not jaundiced
Kernicterus--unconjugated bilirubin crosses the BBB causing cell death and characterized by seizures, hearing loss, poor feeding, and can be fatal
conditions necessary for maternal antibody formation in HDFN (4)
the mother must lack the antigen present on the fetal RBCs
the fetal antigen must be well developed in utero
the mother is exposed to the fetal antigen
the mother produces and IgG antibody to the antigen, capable of crossing the placenta
HDFN antigens
Antigens localized to RBC membrane are more antigenic
Early maturation of antigens plays a role in HDFN severity
e.g. Kell antigen develops early in fetal development
Density of the antigen plays a role as well
Homozygous vs heterozygous expression
HDFN antibodies
IgG crosses from mother's circulation → fetal capillaries in placenta
Neonatal Fc receptor (FcRn) transports IgG from mother to fetus and maintains homeostasis of IgG in circulation
Immunoglobulin transferred to fetus early in 2nd trimester
ABO-incompatible blood group of fetus reduces incidence of maternal formation of anti-D
Group O, Rh neg mothers who carry group A or B, Rh-pos fetuses
ABO incompatible RhD-pos fetal RBCs destroyed and cleared in the mother's circulation before the RhD antigen can be recognized by maternal immune system
ABO HDFN
Before 1968, 95% of HDFN was due to anti-D
Now, most common HDFN is caused by ABO antibody (1:5 pregnancies, mild)
Group O mom with potent high-titered IgG anti-A,B
Infants group A (Caucasian); group B (African-American)
Spherocytes
Bilirubin peaks later (1-3 days)
Mild compared to Rh HDFN
DAT positive
Rh HDFN
Approx 15% of the population is Rh negative (no D antigen)
Rh-negative mothers carrying Rh-positive fetuses run the risk of producing anti-D
Feto-maternal bleeding can occur with any pregnancy
Maternal and fetal blood may mix at delivery
In the mid-1960s, it was discovered that if non-sensitized Rh-negative persons received intramuscular doses of Rh-immune globulin (RhIG), their immune systems failed to produce anti-D antibodies (even after exposure to the D antigen)
Rh-negative pregnant women are now immunized during and immediately after delivery with RhIG
rhogam treatment of Rh HDFN (general)
must determine quantity of Rh-immune globulin to administer
The feto-maternal bleeding usually less than 30 ml of whole blood
One regular-dose vial of RhIG contains 300 ug of RhIG → sufficient to protect against 30 mL of whole blood or 15 mL of packed RBCs
One unit of RhIG is generally sufficient during pregnancy, 28 weeks gestation
The second dose is administered within 72 hours of delivery
In incidences of increased FMH bleeding, the quantity of RhIG units must be calculated to prevent formation of antibodies
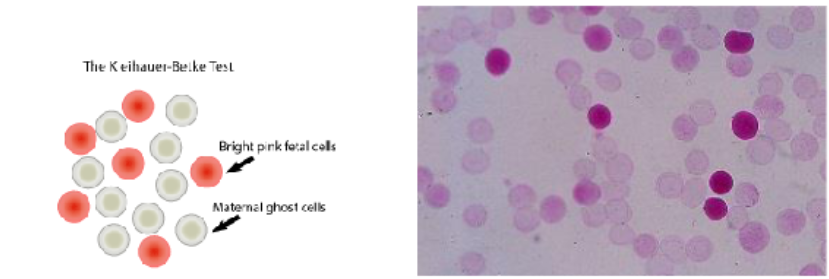
kleihauer-betke acid-elution test
Quantitates fetal cells in the maternal circulation by using a blood smear made from blood drawn from the mother
Smear is placed in an acid buffer
Hemoglobin in adult RBCs (Hgb A) leaches out of the cells into the buffer
Fetal Hgb (Hgb F) is resistant to the acid and will stay within the RBC
Smear is washed, stained with Wright stain, and examined microscopically under oil immersion (1000x)
Adult RBCs will look "ghostlike" because only the cell membrane remains, and the fetal cells will stain pink
kleihauer-betke acid-elution test calculation
determines the % of fetal cells
The number of fetal cells counted per 2000 maternal cells is quantitated similar to a reticulocyte count:
% fetal cells = (# of fetal cells counted / 2000 maternal cells) x 100

rhogam dose calculation
Units or vials of RhIG to be administered are calculated by the following formula:
vials of RhIG = (% fetal cells x 50) / 30
% fetal cells = number of fetal cells per 2000 maternal cells
50 = factor to account for average maternal blood volume
30 = quantity of fetal-maternal bleed
Result is rounded to the nearest whole number
Additional unit is added for safety
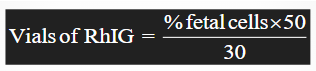
rhogam prophylaxis
Antepartum prophylaxis
300-ug dose of RhIG between 28 and 30 weeks in pregnant Rh-negative women who have not made anti-D
Postpartum prophylaxis
300-ug dose of RhIG within 72 hours to Rh-negative women who deliver Rh-positive infant
Have not produced anti-D
non-ABO HDFN prenatal evaluation
Maternal history involves review of any previous infant affected by HDFN and infants who required transfusion
Serological studies include ABO, Rh, and antibody screen testing (if required)
Paternal phenotype
Likelihood fetus carries antigen to which mother has formed antibody
Typing of fetus
Obtaining a sample of RBCs from fetus can be risk to fetus and/or mother
Amniocentesis procedure of choice to obtain amniotic cells containing fetal DNA
Fetal DNA can be isolated from maternal plasma
HDFN monitoring using antibody titration
Pregnant women w history of anti-D or any other IgG antibody will have baseline titer performed during the first trimester
Recommended to freeze aliquots for future titers every 4-6 weeks during pregnancy
1:2 dilution series of patient serum w saline, 37 C, IAT, anti-IgG reagent
An increase in new titer by 2 dilutions or higher compared w original sample is considered significant
measurements of severity in HDFN
Amniotic fluid analysis
Level of bilirubin pigment in amniotic fluid correlates with hemolysis of fetal RBCs in utero
Doppler flow studies
Ultrasound noninvasive method of monitoring anemia in fetus
Fetal blood sampling
Most accurate means of determining severity of fetal hemolytic anemia and need for treatment of fetus
antepartum treatment of HDFN
Plasma exchange can be safety performed in pregnant women
Goal of removing maternal alloantibody from circulation
Intrauterine transfusion (IUT) or direct intravascular fetal transfusion may be performed as early as 20th week of gestation
Selection of RBCs important
Group O Rh-negative lacking the offending antigen
Less than 5-7 days old (or washed)
Leukocyte-reduced (LR) w CMV-safe, irradiated, hemoglobin S negative and compatible with mother's plasma
intrauterine transfusions (IUT)
Blood must be compatible w maternal antibodies capable of crossing the placenta
Test for maternal antibodies with donor antigens
Ensure no feto-maternal ABO or Rh incompatibility
Crossmatch testing is performed using the mother's sample
serological testing of newborn (cord blood)
Direct antiglobulin test (DAT)
If mother has significant antibody, DAT performed on newborn's sample with anti-IgG reagent
Rh-positive baby born to mother who received RhIG may have positive DAT due to passive transfer of anti-D
If neonate has positive DAT and mother has negative antibody screen, antibody to low-incidence antigen may be suspected
ABO and Rh determination
Only typing of RBCs is required
treatment of HDFN (after baby is born)
Phototherapy
All infants monitored for development of jaundice
Phototherapy or light-based treatment alone
Small volume transfusion
Depends upon degree and rate of hemolysis and resultant increase in bilirubin level
Exchange transfusion
Replacing one or more volumes of infant's blood with compatible RBCs and plasma
Removes antibody-coated RBCs of infant
Removes maternal antibody present in infant's circulation
Corrects anemia without causing volume overload
neonatal transufsions
blood for an exchange or regular transfusion of a neonate should be compatible with any maternal antibodies that may have entered the infants' circulation and optimally react at 37 C or AHG
Testing considerations
ABO and Rh testing required
ABO group of neonate determined only by typing RBC w commercial anti-A and anti-B
Antibody detecting testing required
Indications for red blood cell transfusion
Any baby showing signs or symptoms of anemia
RBC testing for neonatal transfusion
RBC transfusions in infants less than 4 mo of age
Compatibility testing
ABO and Rh type of RBCs from cord/heel stick
Antibody screening on serum or plasma of mother or on eluate prepared from infant's RBCs
Crossmatch compatible w serum or plasma of mother
Stored RBCs for small volume infant transfusions, group O Rh-negative
Leukocyte-reduced, preferably by pre-storage filtration, fresh (5-7 days)
Irradiated, CMV-safe, hemoglobin S negative
Irradiated cellular blood components indicated for infant at risk of transfusion-associated graft vs host disease (TA-GVHD)
Cytomegalovirus (CMV) infection can be devastating disease in fetus or newborn
prevention of Rh HDFN
use of Rh-immune globulin (RhIG)
Decreased incidence of HDFN resulting from production of anti-D by Rh-negative mother
Sterile solution containing IgG anti-D
ABO vs Rh HDFN
ABO
first pregnancy: rare
canNOT predict by titer
main ABY: anti-A,B
billirubin normal + no anemia at birth
IUT: none
exchange transfusion rare; phototherapy needed
Rh
first pregnancy: rare
CAN predict by titer
main ABY: anti-IgG
bilirubin elevated + anemia at birth
IUT: sometimes
exchange transfusions uncommon; phototherapy needed

immune alloantibodies
produced in response to RBC stimulation via transfusion, transplantation, or pregnancy
naturally occurring antibodies
exposure to environment sources that have similar structures to RBC antigens
passively acquired antibodies
via plasma-containing blood components or derivatives (e.g. IVIG)
autoantibodies
directed against one's own RBCs and all the RBCs tested
clinically significant antibodies
IgG, 37 C/AHG (IAT), reduce survival of RBCs
antibody screen (ABS)
detect clinically significant antibodies in the blood donor and the intended recipient
included in standard prenatal testing to evaluate the risk of HDFN in the fetus and to assess the mother's candidacy for Rh-immune globulin (RhIG) prophylaxis
overall purpose: assess for unexpected alloantibodies or (autoantibodies) that may be clinically significant
(antibody screen methods) tube method
2 drops plasma + 1 drop of Group O reagent screening cells
Use of enhancement media
Testing phases
IS, 37 C, and AHG
Confirm all negative reacting tubes with Coomb's control cells (check cells)
Agglutination visualized as "clumps of cells"
ABO typing/grouping ; Rh(D) typing
Antibody screen and identification
Crossmatching
Antigen typing ; DAT
Enhancement media is often used: LISS, PeG, and albumin
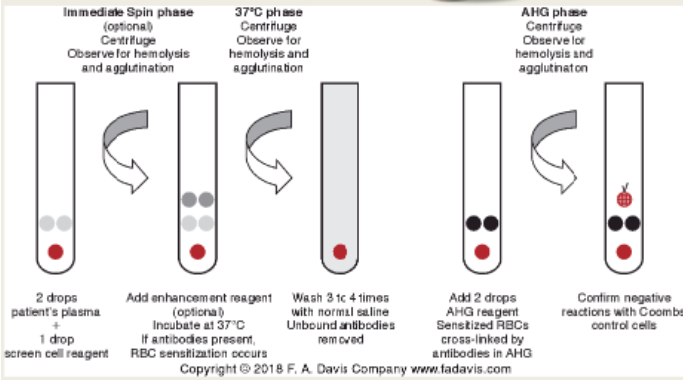

(antibody screen) tube testing methodology
Traditional/gold standard of blood bank testing
IgM (immediate spin or cold reactive antibodies; see first pic)
IgG (indirect antiglobulin test/IAT; second pic)
antigen profiles (reagent rbc panel)
Antigen profiles (antigrams)
Grids that indicate antigen make up of commercial RBCs used for antibody detection or identification
Uses group O reagent RBCs (phenotyped)
A well-designed panel will identify most commonly encountered antibodies
Antibody screen—2 or 3 cell panels
Antibody identification—can be 10, 16, or 20 cell panels
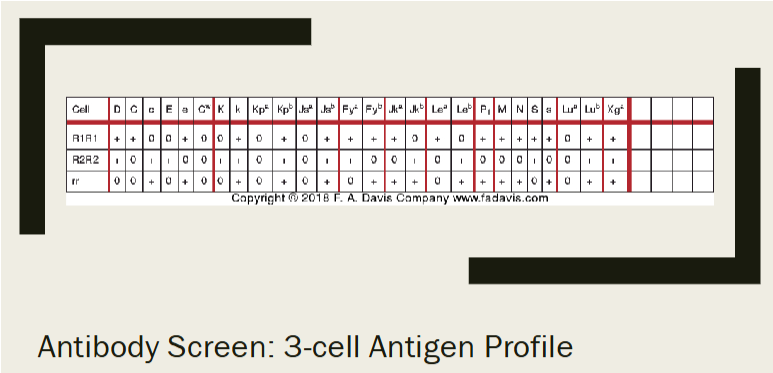
anatomy of antigram
"+" sign refers to the presence of the antigen
"0" sign refers to the absence of the antigen
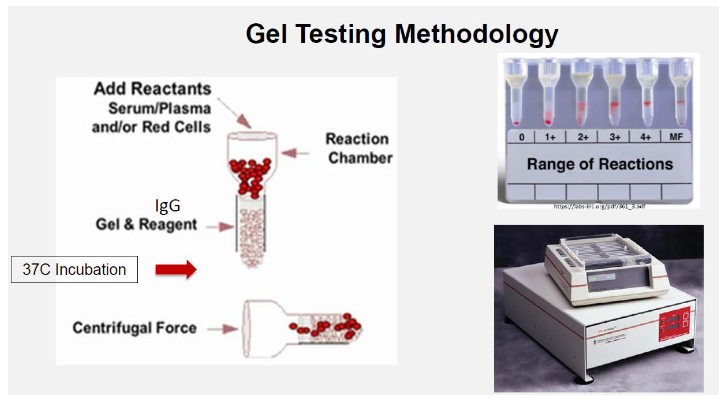
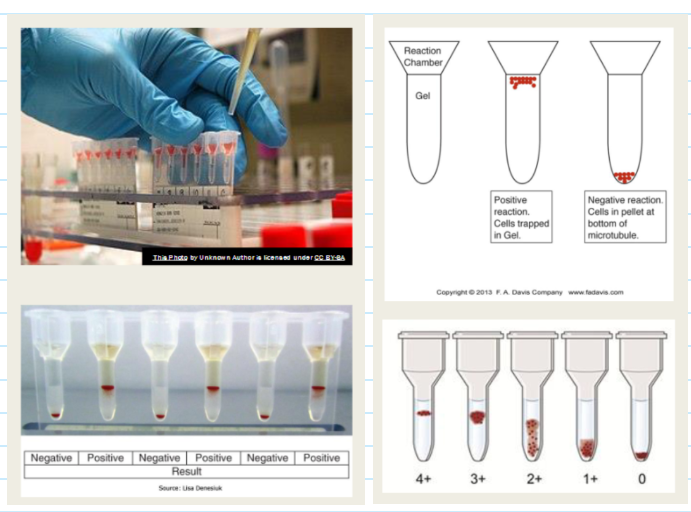
(antibody screenmethods) column agglutination technology (CAT) gel
Agglutination visualized by capture of RBCs within gel column
Used for same routine tests as tube testing
involves microtubules filled with a dextran acrylamide gel
The screen cells used for this technique meet the same criteria as for the tube test BUT are suspended in LISS to a concentration of 0.8%
Large agglutinates remain near or on top of the gel matrix
Smaller agglutinates pass through the gel matrix
Unagglutinated cells pass through the gel matrix to form a button at the bottom of the microtubules
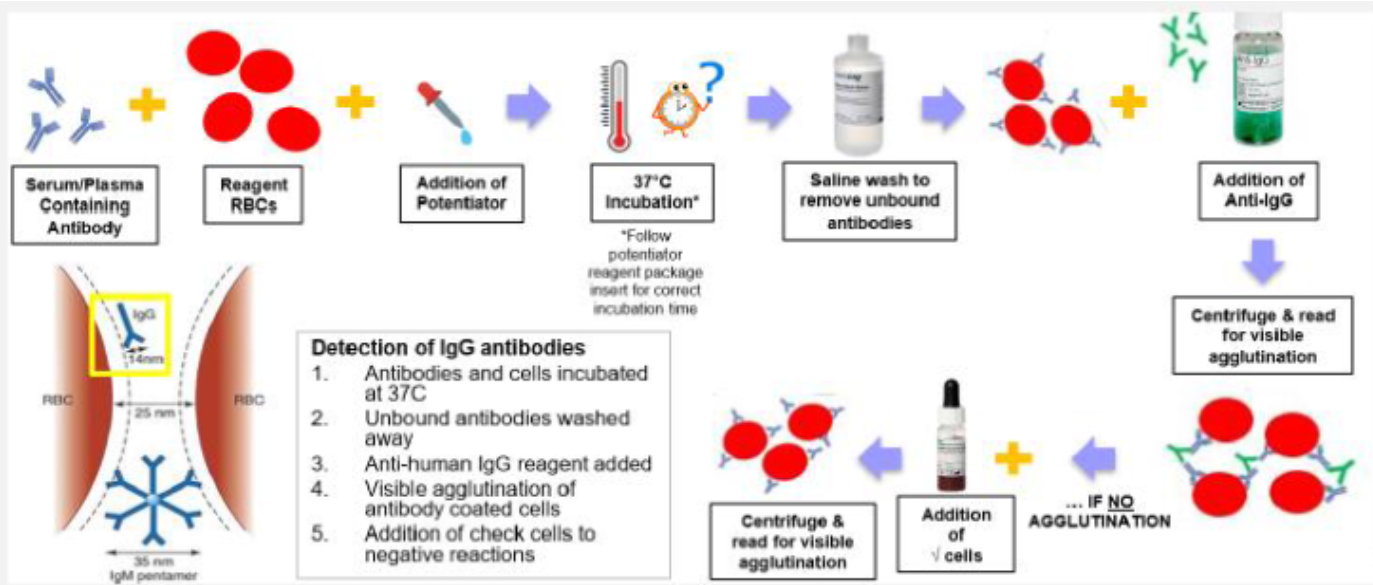
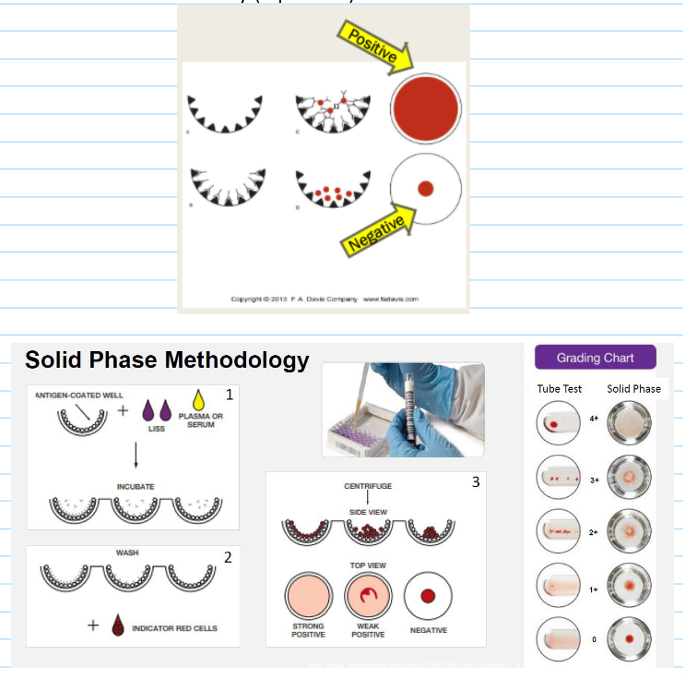
(antibody screen methods) solid phase red cell adherence (SPRCA)
Used for antibody screening and antibody identification
Relies on antigen-coated wells in solid phase microplates
Can be manually performed or on an automated analyzer (Immucor Neo, Immucor Echo)
Testing Process:
Screening cells bound to surface of polystyrene microplates
Pt plasma and LISS added to the wells
RBC antigens capture IgG ABYs during incubation step
Microplates washed to remove unbound ABYs
Indicator cells (or anti-IgG coated red cells) added to wells
Microplates centrifuged to bring indicator cells in contact with bound antibody (if present)
pros/cons of tube method for antibody screen
detects IgG & IgM; needs 100 uL of plasma/serum
uses 3-5% suspension of RBCs
advantages:
gold standard, easily manipulated, differentiates IgM from IgG
disadvantages:
no automation available, human techniques and subjectivity, unstable rxns
pros/cons of gel method for antibody screen
detects IgG & IgM; needs 25 uL of plasma/serum
uses 0.8% suspension of RBCs
advantages:
small sample volume, very sensitive, stable rxns, automation available
disadvantages:
no wash step, IgM interference in IgG tests
pros/cons of solid phase method for antibody screen
detects IgG only; needs 50 uL of plasma/serum
uses 3% suspension of RBCs
advantages:
automated, sensitive, designed for IgG, stable reactions
disadvantages:
very sensitive to autoantibodies, must prepare own monlayers or buy commerically prepared
autocontrol (AC)
An autologous control consists of testing the patient's serum (or plasma) against their own RBCs by the same method used to test ABID panel cells
Tested whenever an ABID panel is performed
2 drops plasma or serum + 1 drop RBC suspension
Autoantibodies, when present, may complicate the detection of clinically significant alloantibodies
Hence the setup of testing an autocontrol
questions to ask to interpret antibody screens
In what phase(s) did the reaction(s) occur?
Is the autologous control negative or positive?
Did more than one screen cell react?
If so, did they react at the same strength/phase(s)?
Is hemolysis or mixed-field agglutination present?
Hemolysis? Anti-Lea, anti-Leb, anti-PP1Pk, anti-Vel
MF? Lutheran, anti-Sda
Are the cells truly agglutinated or is rouleaux present?
Does not interfere with AHG phase in tube or solid-phase testing due to patient’s serum being washed away prior to addition of AHG
Saline wash
limitations of an antibody screen
Will not detect ALL antibodies!!
If a certain Ag (low prevalence) is not represented on screening cells, the Ab (if present) will not be detected
Antibodies that show dosage
Antibodies with low titers
Screen may look negative; antigen positive units given => result in delayed hemolytic transfusion reaction days or weeks later
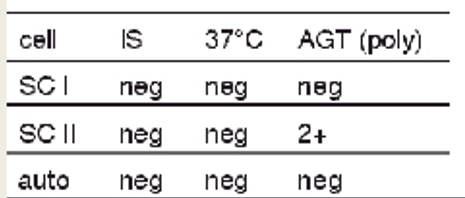
antibody screen results + possible interpretations:
2+ only at AHG phase with SCII
single alloantibody
two alloantibodies, antigens only present on cell II
probably IgG alloantibody
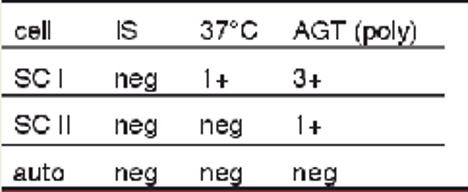
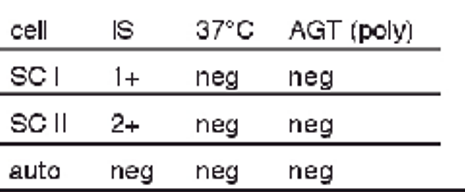
antibody screen results + possible interpretations:
1+ at 37 C with SCI
3+ at AHG with SCI
1+ at AHG with SCII
multiple alloantibodies
single alloantibody (dosage)
probably IgG alloantibody
antibody screen results + possible interpretations:
1+ at IS with SCI
2+ at IS with SCII
single or multiple antibodies
probably IgM alloantibody
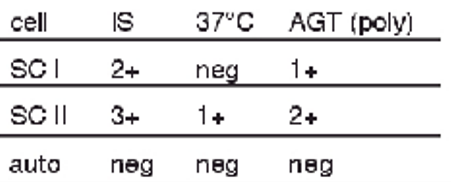
antibody screen results + possible interpretations:
2+ at IS w SCI
1+ at AHG w SCI
3+ at IS; 1+ at 37 C; 2+ at AHT w SCII
multiple alloantibodies, warm and cold
potent cold alloantibody binding complement in AHG
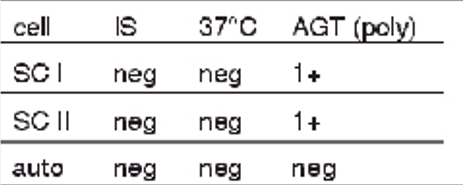
antibody screen results + possible interpretations
1+ at AHG w SCI and SCII
single warm alloantibody, antigen present on both cells
antibody to high-prevalence antigen
complement binding by a cold alloantibody not detected at IS
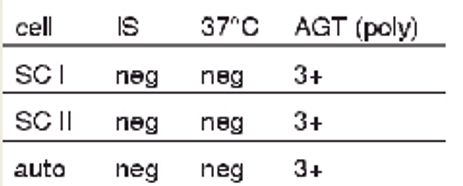
antibody screen results + possible interpretations
3+ at AHG for SCI, SCII, and with autocontrol
warm alloantibodies
transfusion reaction
probably IgG alloantibody
warm autoantibody
what is done after a positive antibody screen?
reflex to ABID panel for identification of antibodies using IAT procedure
Patient serum or plasma mixed with Group O Screening Cells (RBCs) with known antigens
Test phases and enhancement reagents
Tube, gel, and solid phase methods are approved for ABID testing
Gel and solid phase testing are very sensitive techniques
Tube testing has more variation in technique but still helpful in identifying antibodies
purpose of ABID
Performed to determine the specificity of the antibody/antibodies
Single alloantibody may be simple to ID
Multiple alloantibodies or autoantibodies may be complex and require a great deal of time and expertise
Important when selecting donor units for transfusion
Monitoring potential HDFN cases
how is an ABID panel done?
Testing of plasma against panel of 11 to 20 group O RBCs with KNOWN phenotypes
Focused on DETECTION of unexpected antibodies
Each panel should have antigram with various antigen expression
Shows antigenic makeup of each cell in panel
Should include cells with homozygous expression
what patient history is relevant to know for an ABID panel?
Has patient ever been transfused or pregnant?
If patient has been transfused, did any transfusions occur in the last 3 months?
What medication is the patient currently taking?
Are there any other medications patient has taken in the last 3 months?
ABID panel selection
The purpose of the ABID panel is to identify unexpected clinically significant antibodies
Antibody screen and ABID panel cells are prepared from group O donors
Cells do not contain A or B antigens on RBC surface
Eliminates chance of interference from ABO antibodies in serum or plasma
Factors influencing sensitivity
Serum to cell ratio
Temperature and phase of reactivity
Length of incubation and pH
difference between antibody screen & antibody identification
Screening uses two to three different donor cells
Antibody identification uses cells from a larger number of donors (10 to 20 donors)
(ABID panel evaluation) questions to ask for inital interpretation of panel
Where did the reactions occur?
Is the autologous control negative or positive?
In what phase(s) and at what strength(s) did the positive reaction(s) occur?
AHG phase positive? = IgG antibody
All same strength? = single antibody
Did more than one panel cell react?
If so, did they react at the same strength/phase(s)?
In tube method, IS phase positive only = COLD IgM; not clinically significant!
If positive in all three phases; may have two antibodies (cold and warm) or a cold with wide thermal range
(ABID panel evaluation) questions to consider after ruling out antibodies
Did positive antibody reactions match a pattern of any antigens that were not ruled out?
If single alloantibody present, the pattern of reactivity may match exactly
If not an exact match, consider:
Dosage
Multiple alloantibodies
Cold reactive autoantibodies
Antibodies directed against high frequency or low frequency antigens
Were all commonly encountered RBC antibodies excluded?
Low-prevalence antigens
Was there sufficient evidence to prove the suspected antibody(ies)?
3 and 3 rule
Does the patient lack the antigen(s) corresponding to the antibody(ies)?
optimal phases of reactivity for various antibodies
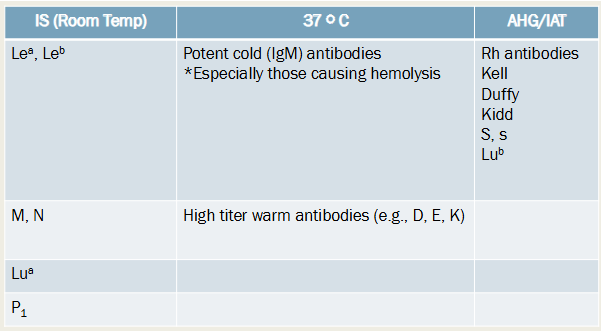
IS (RT): Lea, Leb; M, N; Lua; P1
37 C: potent cold (IgM) antibodies esp those causing hemolysis; high titer warm antibodies (e.g., D, E, K)
AHG/IAT: Rh antibodies, Kell, Duffy, Kidd, S,s; Lub
exclusion/”rule out” general rules
only screening/ID panel cells that gave NEGATIVE reactions with patient plasma in ALL phases should be used to rule out
Dosage—rule out using panel cells with HOMOZYGOUS (double dose of antigen) present
e.g., Rh (except D), Duffy, Kidd, MNS, and Lutheran
Antibodies that react more strongly with cells having homozygous antigen expression are said to “show dosage”
After each negatively reacting cell has been evaluated, the remaining antigens should be examined to see if the pattern of reactivity matches a pattern of antigen-positive cells (inclusion technique)
exclusion/”rule out” exceptions
There are exceptions to rule advocating use of only homozygous cells for exclusion of antibody
e.g., D and P1 antigens do not have antithetical alleles
Careful exception: you may rule out potential anti-K with heterozygous Kk ag
Antibodies to low frequency antigens (less than 2-5% of population) are uncommon
e.g., Anti-Cw, Anti-V, anti-VS, anti- Lua, anti-Jsa, anti-Kpa
Most of the time you do not need to actually rule them out
exclusion/”rule out” rules for commonly encountered antibodies
may need to use additional panel cells for more evidence to “rule in” or “rule out”
Select panel cells have homozygous antigens expression corresponding to your suspected antibody
If you have multiple antibodies, the select panel cells will also need to be negative for the antigens for the other antibodies in question
statistical measures for ABID
Probability or p value: statistical measure that predicts likelihood of an outcome
To be certain 95% of the time:
Rule of Three: antibody must react with three (3) antigen-positive cells and must not react with three (3) antigen-negative cells to be statistically proven
(techniques for resolving ABID) selected cell panels
The cells selected for testing should have minimal overlap in the antigens they possess
Selected cell panels are also useful when a patient has a known antibody, and the blood bank is attempting to determine if additional antibodies are present
(techniques for resolving ABID) antigen phenotyping
Determines phenotype of tested RBCs
Helps prove antibody present (Does the patient RBC lack the antigen corresponding to the alloantibody in the plasma?)
Rules out antibodies not present (Patients will not make an alloantibody against an antigen that they possess!)
Special consideration: if transfused within the last 3 months or has a positive DAT
Test specific known antisera against patient or donor RBCs
summary of the steps of antibody identification
Positive antibody screen
Next, test serum/plasma against a panel of reagent red cells
Tube--RT, 37 C and AHG phases/CCC
Gel and solid phase = 37 C/AHG
Perform "ruling out" technique
Test selected cell panel
Antigen phenotyping
Patient red cells
Donor red cells: should be antigen negative for antibody determined!