Chem PPQs got wrong topic 5
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
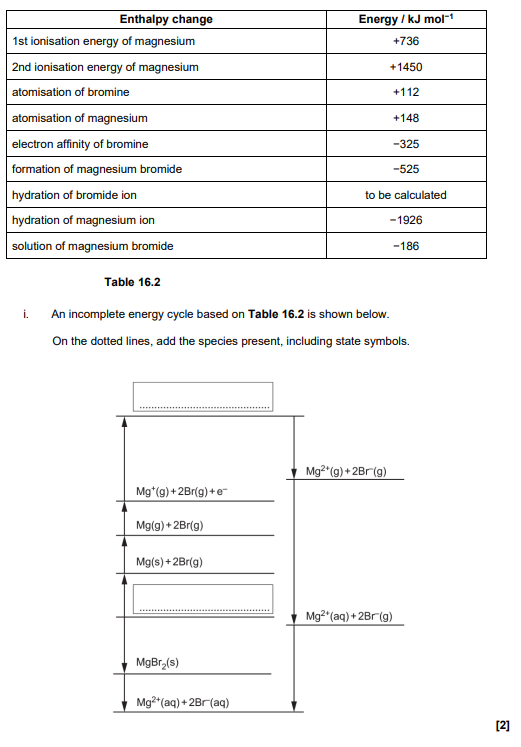
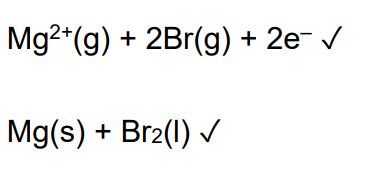
The lattice enthalpy of sodium oxide is more exothermic than of potassium oxide explain why (2)
Comparison of size of cations
for sodium ions ionic radius of sodium is smaller
Comparison of attraction of cation and anion
~Na+ has stronger attraction to O2-
Explain what is meant by the term enthalpy change of hydration (2)
the enthalpy change when
1 mole of gaseous ions react OR 1 mole of hydrated / aq ions are formed
gaseous ions dissolve in water OR gaseous ions form aq / hydrated ions
Predict how the enthalpy changes of hydration of F- and Cl- would differ (2)
enthalpy of hydration of F- is more negative / exothermic than enthalpy of hydration of Cl- AND F- has a smaller size
F- OR smaller sizes ion linked to greater attraction TO H2O