lecture 5 and 6- pharmacokinetics
1/64
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
65 Terms
pharmacodynamics
what a drug does to the body
pharmacokinetics
what the body does to a drug
why is pharmacokinetics important in drug discovery
in the clinic you can see individual variations within populations and ethnic groups in how they respond to drugs, so the dose and frequency of administration needs to be adjusted to ensure control of the amount of drug in the body is within the ‘therapeutic window’
in pharmacodynamics, physiochemical properties of the drug affects…
affinity, efficacy and potency
in pharmacokinetics, the physiochemical properties of the drug affects…
absorption, distribution, metabolism and excretion
as the concentration of the drug increases…
the effect increases (for most drugs)
the time it takes for the drug to start working, reach its maximum effect and then wear off, directly mirrors…
how the amount of drug at the specific location where it acts changes over time (with the exception of irreversibly bound drugs)
distribution is from…
the bulk flow transfer through the bloodstream to take the drug to its target and to its sites of elimination
what is the diffusion coefficient (for the absorption of a drug) proportional to
1/sqrt(MW): the smaller the drug, the higher the diffusion coefficient so the faster the drug diffuses
what determines where and for how long a drug will be present in the body after it has been administered
the movement between compartments, generally involving penetration of non-aqueous diffusion barriers
what are some types of barriers a drug might diffuse through
plasma membrane
vascular endothelium
the CNS and placenta
pinocytosis
a process where the cell takes in extracellular fluid and dissolved small moleceules by engulfing them with its membrane
process of pinocytosis
the cell membrane forms a small pouch around the fluid and solutes, which then pinches off to form a vesicle inside the cell (this is a form of endocytosis)
factors affecting absorption
diffusion through lipids
pH and ionisation
route of administration
transport
the diffusion of drugs through lipids depend on…
the concentration gradient and diffusion coefficient
the concentration gradient in cell membranes will depend on…
the lipid-water partition coefficient (lipid solubility)
what is the advantage of non-polar drugs
they are easy to eliminate
many drugs are weak acids or bases:
HA ⇌ H+ + A-
the Henderson-Hasselbalch equation:
pKa=pH + log10(HA/A-) or pKa=pH +log10(BH+/B)
at low pH, weak acids will be…
unionised
weak acid
a molecule that can give up a H+
pH affects steady-state distribution of drugs between…
aqueous apartments
weak acids become trapped in…
basic compartments
where is the main site of absorption in the body
the small intestine (not stomach) due to its enormous absorptive surface area (surface area overrides determinant of the site of the drug)
give an example of how pH affects drugs
drugs like metoclopramide (speeds up gastric emptying) promote aspirin (weak acid) absorption
drugs like propantheline slow down aspirin absorption
weak acids will accumulate in compartments with…
high pH
intravenous route
most direct, fast acting, used in emergencies
IV treatment, goes directly within the vein
intrathecal route
the substance is directly injected into the spinal canal which contains cerebrospinal fluid
used when a high/ rapid amount of a drug is required to reach the CNS, mainly used in childbirth
oral/ rectal route
most common, most drug absorption occurs in the gut by passive diffusion (rate of absorption will depend on gut motility, presence of food, particle size of the tablet, encasing of the tablet and physiochemical factors)
sublingual administration
when the drug is placed under the tongue to be absorbed
percutaneous route
includes skin patches, cornea eye drops, nasal mucosa cold remedies and some peptide hormones (injected under skin)
useful when you want a local affect but most drugs do not penetrate very well unless they are lipophilic
inhalation route
restricted to gases used for general anaesthetics and for local effects like administration of bronchodilators in asthma
intramuscular route
vaccinations, done deeper than percutaneous so it has better administration and distribution
soluble carrier transporters (SLC) are associated with…
passive or secondary transport
the main sites of solute carrier transporters are:
organic cation transporter (OCTs) and organic anion transporters (OATs) including:
the blood brain barrier
the gastrointestinal tract
the renal tubule
the bile tract
the placenta
ATP-binding cassette transporters (ABC)
assoicated with primary active transport
OCTs-uniporters- OCT2
transporter of proximal tubular cells in kidneys and concentrates drug such as cisplatin (anticancer drug)
drug transporters usually have extensive binding affinity towards…
a broad spectrum of small molecule substrates and inhibitors
genetic variants of OCT1 (found in liver cells) are associated with…
different responses to metformin (lowers blood sugar) in healthy humans
OCT1-variant is __ effective at lowering blood sugar
less
most drugs do not spread rapidly throughout all body water compartments to acheieve a uniform concentration at equilibrium, the concentration in each compartment will depend on:
permeability across tissue barriers
protein binding within compartments
pH partition
fat: water partition
why may drug distribution in the CNS be limited by the blood brain barrier
the endothelial cells lining the blood vessels in the CNS form tight junctions impermeable to water soluble moloecules
what does inflammation in the blood brain barrier cause
the blood brain barrier becomes leaky, so drugs like penicilin can reach the blood brain barrier CFS and treat things like meningitis
chemoreceptor trigger zones also become leaky which is useful in counteracting nausea associated with some drugs
infections compromise the blood brain barrier so antibodies can…
easily pass through
body fat acts as a
drug reservoir
tetracyclines accumulate in bones and teeth because of…
high affinity for calcium (problem in children)
phase 1: catabolic reactions
changes the nature of the drug to make it more reactive
phase 2: synthetic (anabolic reactions)
changes the chemical nature of the drug to make it inactive and to facilitate its secretion
where and how are drugs mediated
mediated in the liver using microsomal enzymes- the drug must first cross the plasma membrane to be metabolised
elimination of aspirin
aspitin → salicylic acid → glucuronide
the salicylic acid is short lived because in phase 2 a sugar is added onto the hydroxyl group (it can no longer bind to its target and can now be excreted)
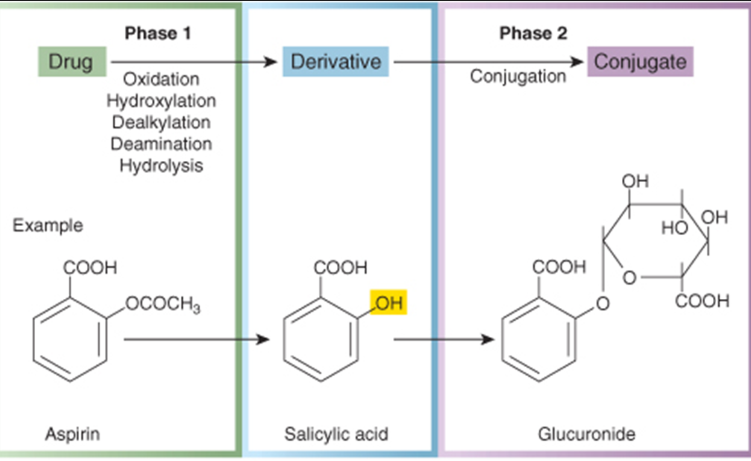
what type of processes happen in phase 1
oxidation, hydroxylation, dealkylation, deamination, hydrolysis
what type of process occurs in phase 2
conjugation
products of the phase 1 reactions may have increased…
toxicity and carcinogenicity
what enzymes carry out phase 1 reactions
the cytochrome p450 family
what kind of environmental factors influence the activity of cytochrome p450
grapefruit juice inhibits p450 (cardiac dyrhythmias)
brussel sprouts and tabacco can induce p450
what happens when you overdose on paracetamol
the phase 1 reaction produces NAPQI which is extremely toxic (in phase 2 it becomes inactive). overdosing on paracetamol can lead to a build up of this toxic intermediate and the body cannot keep up with the phase 2 reactions; it begins to kill liver cells
why is it okay to combine paracetamol with ibuprofen
they use different metabolism pathways
why is the inducer of p450, carbamazepine, a problem
the p450 cytochrome is also used to metabolise carbamazepine, which affects drug concentration
excretion of drugs via the kidneys happens by 1 of 3 main routes:
glomerular filtration (unless highly protein bound)
active tubular secretion (for weak acids/ bases, uses OAT and OCT transporters)
passive diffusion (for lipid-soluble drugs, inefficient excretion)
what other ways are drugs eliminated from the body
GI excretion and lung excretion
why is predicting time course of drug action very important clinically
it underpins the dosing regime time course of drug concentrations attained in the body during and after dosing
what does first order kinetics show in a graph
the body eliminates the drug at a proportional rate to the drug concentration. the time it takes to reach a steady state is the same for all doses
what does a zero order kinetics graph show
once the elimination pathways become saturated, the drug is eliminated at a constant, fixed rate, regardless of concentration. the drug accumulates much faster and the time to reach steady state is longer
steady state
when the overall rate of drug input into the body is equal to the overall rate of drug output
the maximum rate at which the drug can be administered should be limited by…
the maximum rate at which it can be eliminated