chemistry questions i've asked myself while revising / questions i've gotten wrong
1/85
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
86 Terms

B

C

The solvent evaporates. The oil has changed chemically by reacting with oxygen in the air.

b
As the two states are at the same temperature, the particles have the same average kinetic energy and are moving at the same average speeds.
The inter-particle distances in the gas are significantly larger than those in the liquid.
why can evaporation happen over a range of temperatures
Evaporation can occur over a range of temperatures because particles in a liquid have a range of kinetic energies.
At any temperature, some surface particles have enough energy to overcome intermolecular forces and escape into the gas phase.
Imagine lots of tiny bouncy balls inside a cup of water.
Some balls are moving slow
Some balls are moving super fast
Even when the water isn’t very hot, a few of the super fast balls at the top can:
Jump out of the cup into the air
Those jumping-out balls are called evaporation.
So evaporation can happen any time because:
There are always a few fast ones
And the ones at the top can escape
But when the water is boiling, all the balls are jumping like crazy — that’s different 😊
why does the depth of a liquid not increase the rate of evaporation
Evaporation only happens at the surface of a liquid, not throughout it.
So making the liquid deeper doesn’t give it more surface to evaporate from.

Snow can disappear without melting because ice can sublime, where surface particles with enough energy change directly from solid to gas.
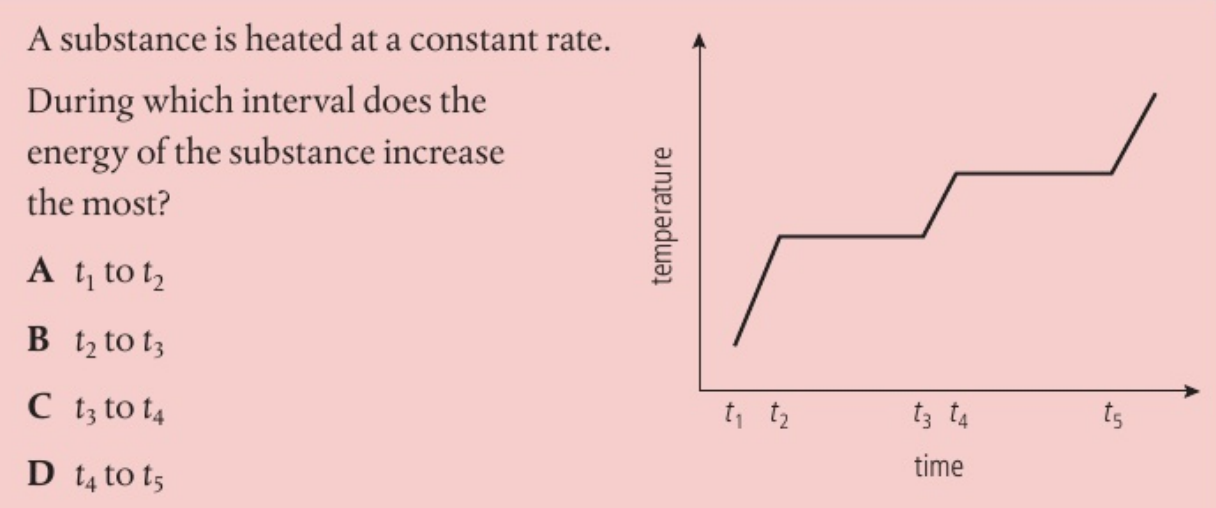
D As the substance is heated at a contant rate the energy increases most during the largest time inteval.
The longest time gets the most energy because the heater adds energy at the same speed the whole time.
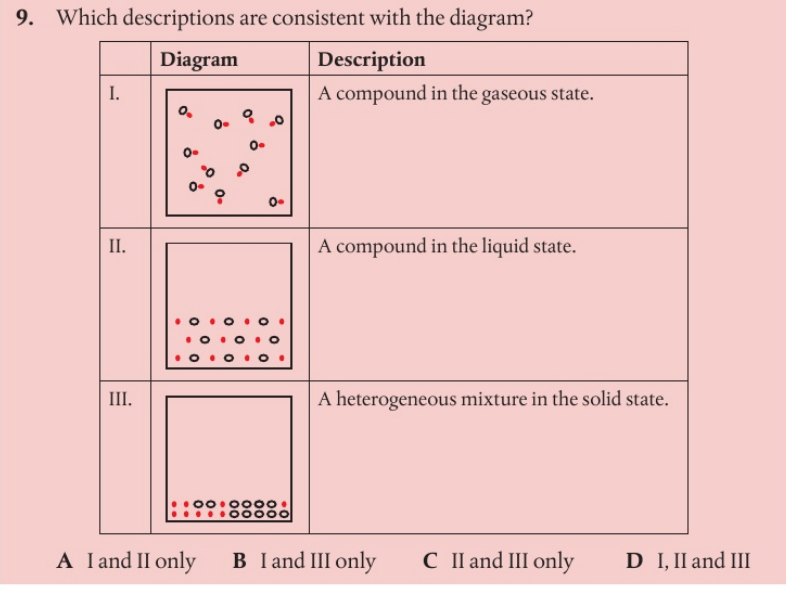
b
are alloys homogenous or heterogenous and why
An alloy is a mixture of two or more elements, at least one of which is a metal.
The different atoms are evenly distributed throughout the metal.
There are no visible boundaries between the substances.
The composition is uniform throughout.
Alloys are homogeneous because their different atoms are evenly mixed throughout and the composition is uniform.

(At certain conditions of low temperature and low humidity), snow changes directly to water vapour by sublimation, without going through the liquid phase.

the gas particles do not have the same speed (1)
the speed of individual particles changes with each collision (1)
this is true all the time
Even at constant temperature, gas particles have a range of speeds because frequent collisions constantly transfer energy between particles.

Technetium, Tc, has no stable isotopes. Note that its relative mass is an integer and given in parentheses.
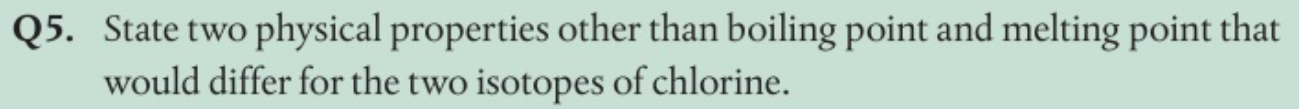
Examples are density (related to mass)
As chlorine is a gas, rate of diffusion.
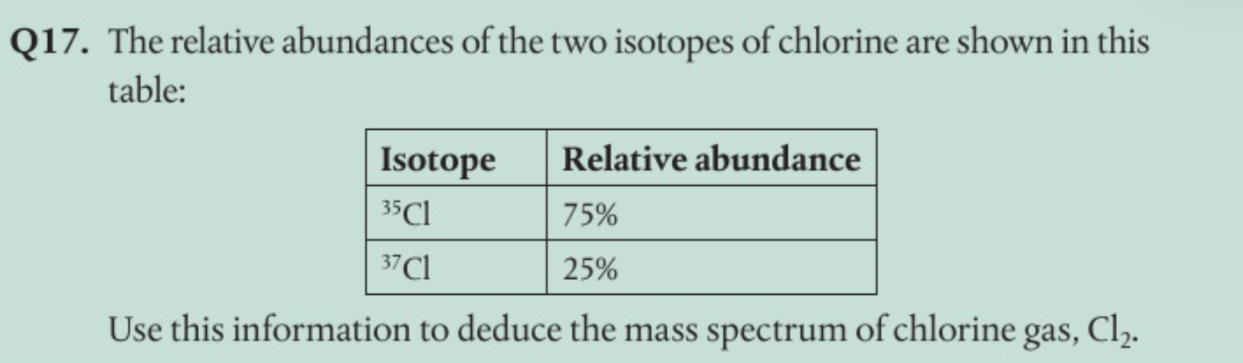
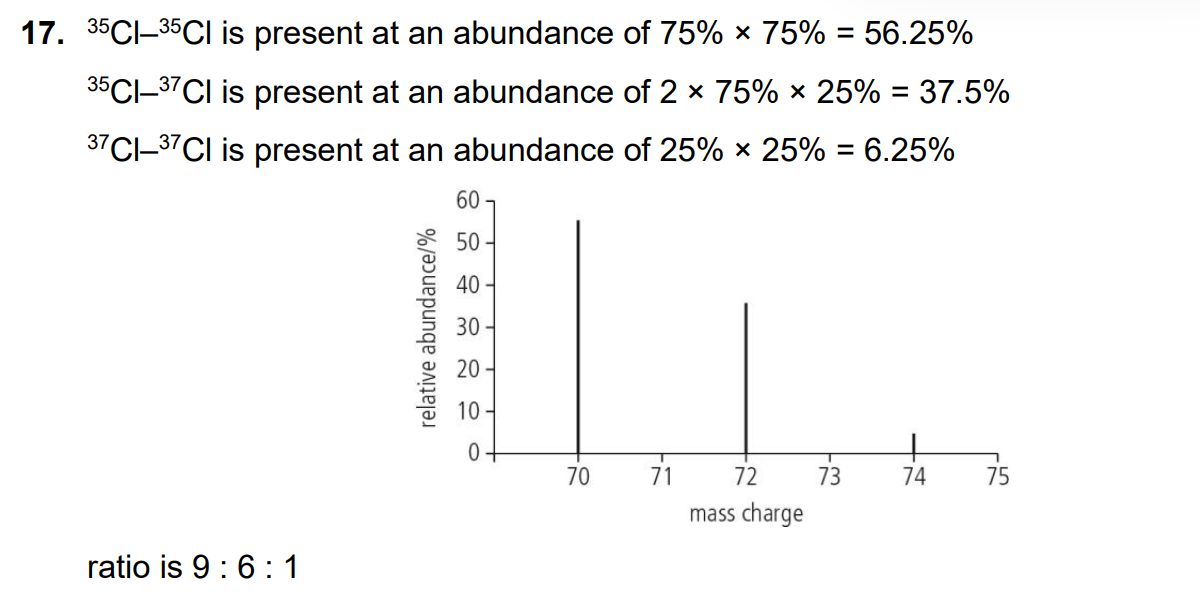
how can two isotopes have the same chemical properties
Isotopes of the same element have the same number of protons → same nuclear charge → same attraction for electrons → same electron configuration.
Since chemical properties are determined by electrons (especially valence electrons), isotopes behave almost identically in chemical reactions.
if chemical properties are determined by electrons, what are physical properties determined by
Physical properties are determined mainly by structure + forces + mass, not by how substances react chemically.
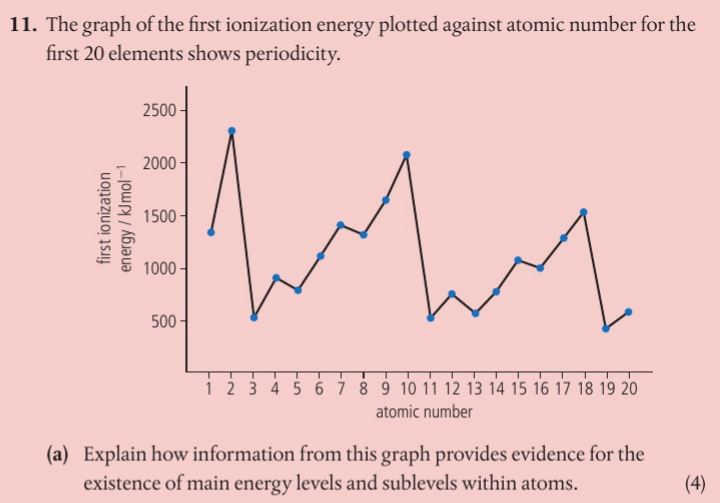
How does the emission spectra provide evidence for the existence of atomic levels
The lines in the spectra are produced by the transition of electrons between atomic energy levels.
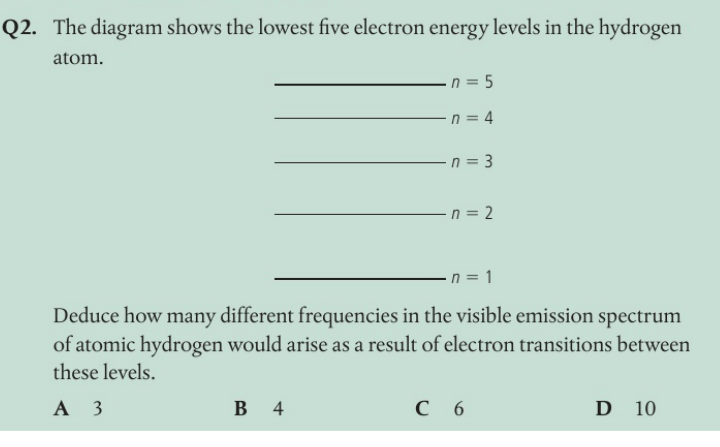
A Visible transitions result in the Balmer series of transitions and are for transitions that end at the n = 2 level. There are three possible transitions: n = 5 to n = 2, n = 4 to n = 2, and n = 3 to n = 2. Any other transitions would fall outside of the visible region of the spectrum.
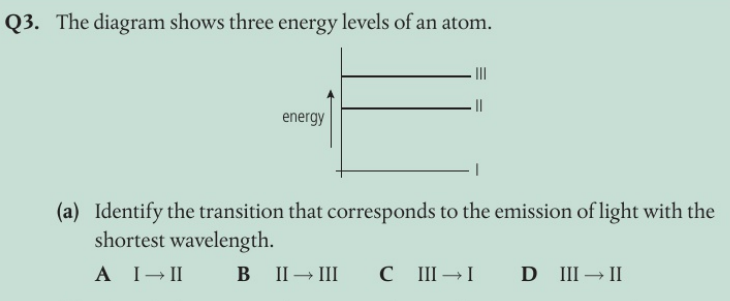
C The shortest wavelength corresponds to the largest energy transition.
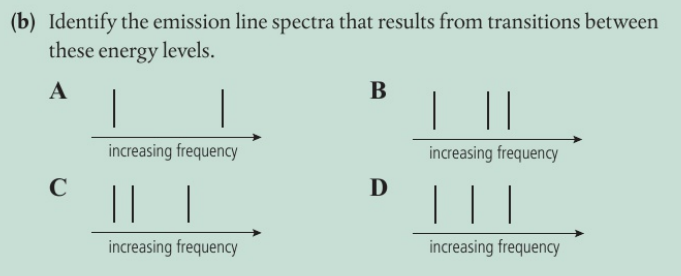
B The lines converge at higher frequency as the energy levels converge at higher energies.

The transition corresponds to a larger energy change in the atom. The emitted photon has more energy and corresponds to a lower wavelength.

Number of orbitals = n 2 = 42 = 16 = (1 × s) + (3 × p) + (5 × d) + (7 × f) = 16
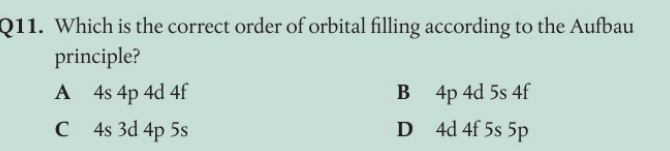
c

B It is unusual to have more than one unfilled set of orbitals. [Ne]3s23p34s1 is the excited state and [Ne]3s23p44s0 would be the ground state. Chromium ([Ar]3d54s1 ) and similar elements have two unfilled orbitals, but these are exceptional, and show the stability of the half-filled d orbital.
When the ions form, it is the 4s electrons which are lost first:
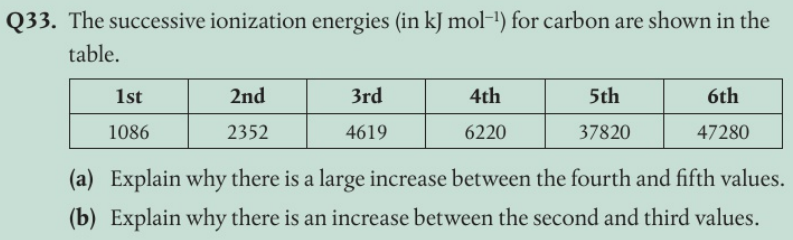
(b)
(b) The 2nd electron is removed from a 2p orbital, the 3rd electron from the 2s orbital. Electrons in 2s orbitals are closer to the nucleus and so experience a stronger electrostatic force of attraction.





x =1
y=5
distinguish between a continuous spectrum and a line spectrum
A continuous spectrum has all colours/wavelengths/frequencies whereas a line spectrum has only lines of sharp/discrete/specific colours/wavelengths/frequencies.

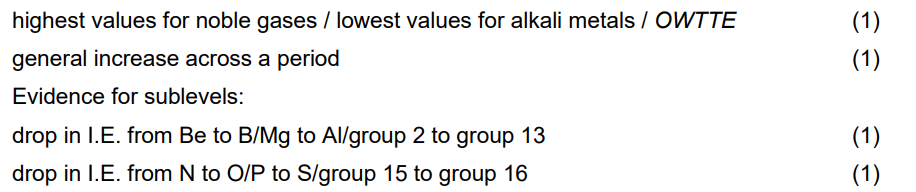

c
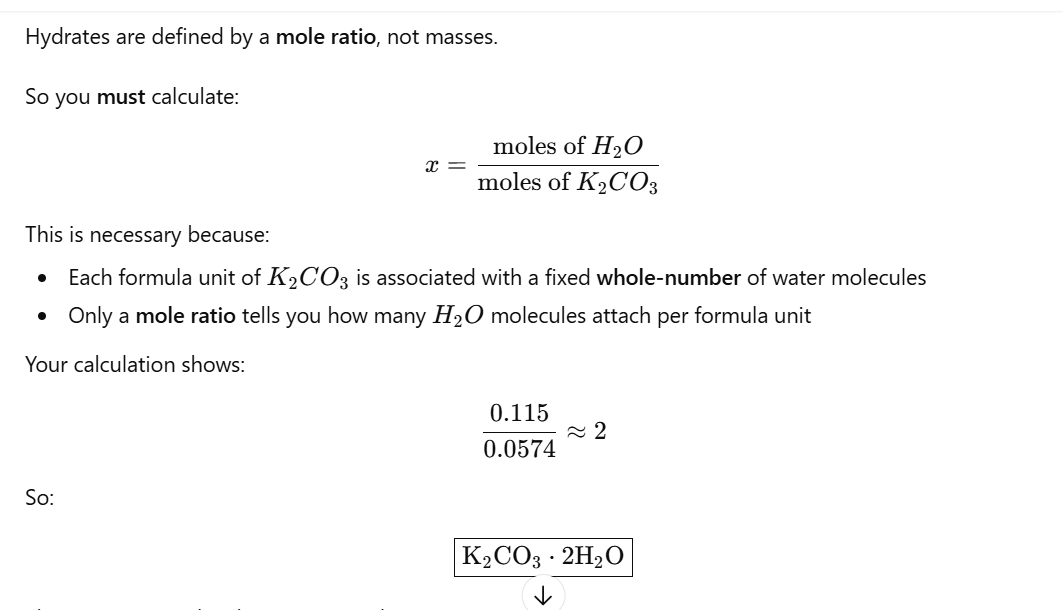


work out moles using conc & volume
n = cv
at low pressure PV/RT is more/less than 1
at low pressure PV/RT is less than 1
What is PV/RT?
PV/RT = Z , a way of measuring how much a real gas deviates from ideal gas behaviour.
Z=1 → gas behaves ideally
Z≠1 → gas is non-ideal (real gas)
What does it mean if PV/RT is less than 1?
If Z<1
PV<nRT
Physically, this means:
The gas occupies less volume than an ideal gas or
The measured pressure is lower than expected for an ideal gas
Why is PV/RT less than one at low pressure?
At low pressure, gas particles are far apart, so intermolecular attractions reduce the effective pressure, causing the real gas to occupy a smaller volume than predicted for an ideal gas.
at high pressure PV/RT is more/less than 1
at high pressure PV/RT is more than 1
What does it mean if PV/RT is more than 1?
if Z>1
PV >nRT
Physically, this means:
Intermolecular repulsive forces dominate
Therefore:
The gas occupies more volume than an ideal gas, or
The measured pressure is higher than expected for an ideal gas
why is PV/RT > more than one at high pressure
At high pressure, gas particles are very close together, so repulsive forces between them push them apart. This reduces collisions with the walls less than expected, making the pressure higher than ideal,
how to calculate volume of a gas using 22.7dm3 and mols
when is this calculation used?
volume = mols x 22.7dm3
only at STP 273K and 1atm
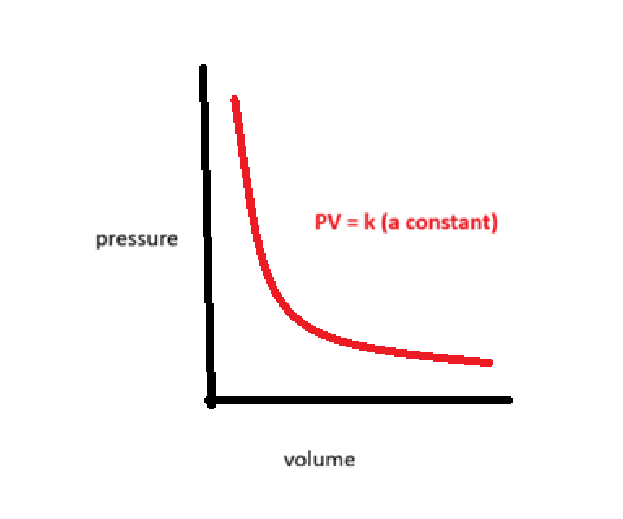
what is the relationship between pressure and volume
boyles law
the volume occupied by a gas is inversely proportional to it’s pressure
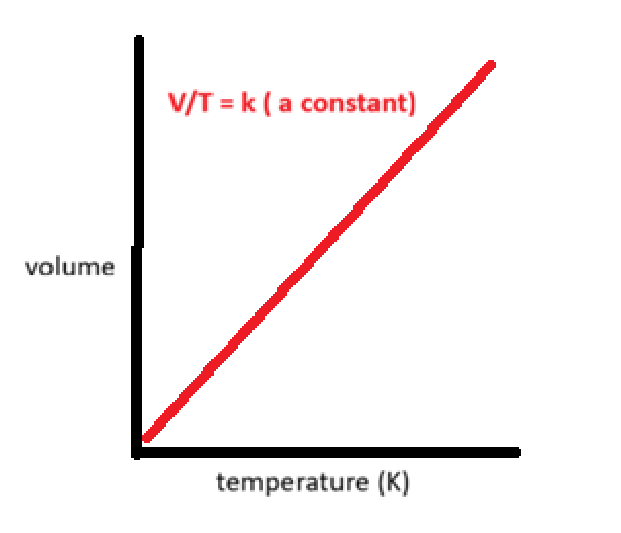
what is the relationship between volume and temperature
charles’ law
the volume occupied by a gas is directly proportional to it’s absolute temperature in Kelvin
what is the relationship between pressure and temperature
gay lussacs law
the pressure exerted by a gas is directly proportional to it’s absolute temperature in Kelvin at constant temperature
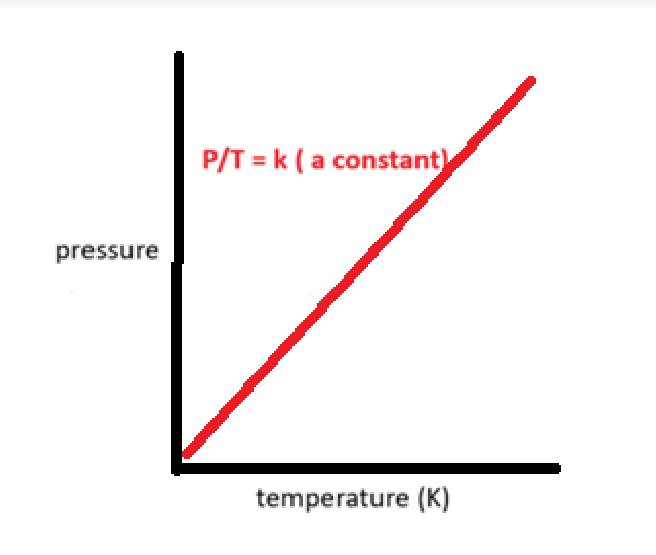
what is the relationship between volume and mols
avogadro law
The volume occupied by a gas is directly proportional to the amount (in mol) of gas at constant pressure and temperature
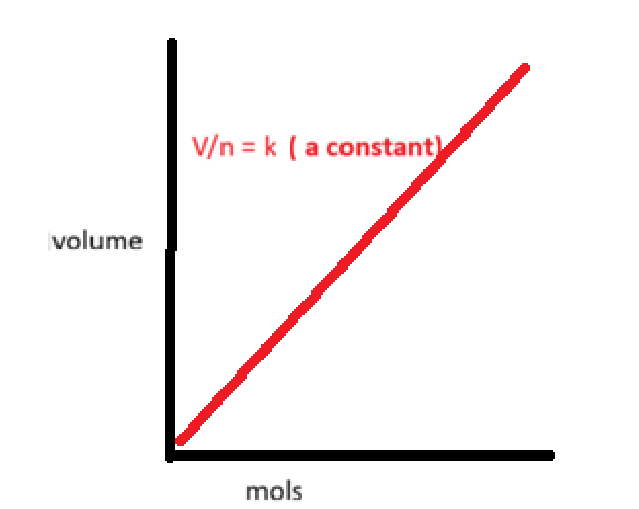

why is the answer D
Pressure comes from particles colliding with the walls of the container, not from collisions with each other.
what is a monatomic gas
gas composed of single atoms that are not bonded to each other.
The volume V for a fixed mass of an ideal gas was measured at constant temperature and different pressures P. Draw the graph that shows the correct relationship.
On the y axis is PV and on the x axis is V
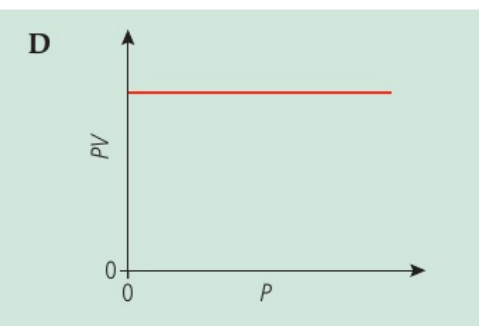
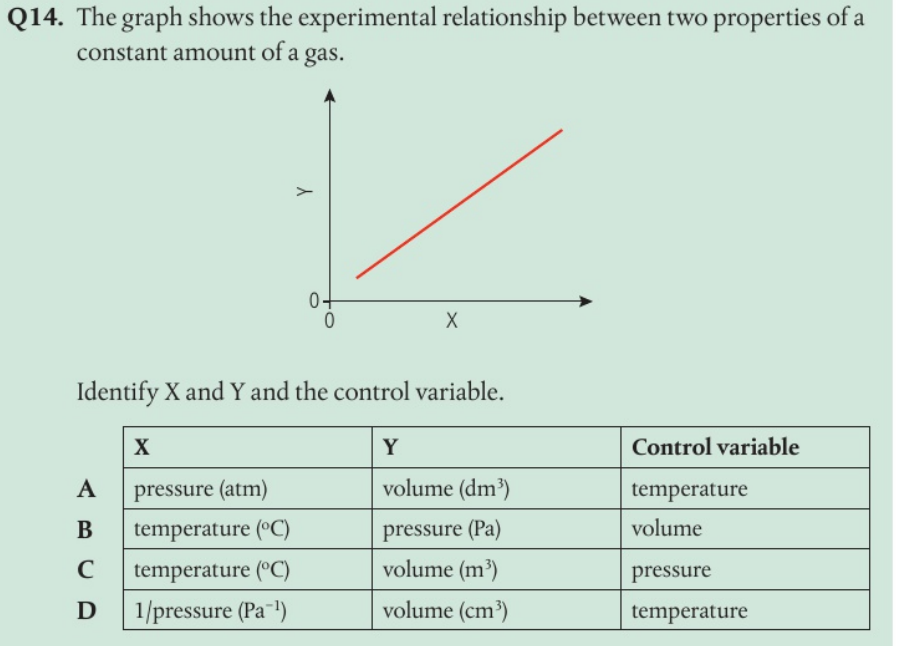
D
An unknown noble gas has a density of 5.84g dm³ at STP. Calculate its molar mass, and so identify the gas.
3dm3
A cyclist pumps his tyres up very hard before a trip over a mountain pass at high altitude. Near the summit one of his tyres explodes. Suggest why this may have occurred.
At higher altitude the external air pressure is less. As the air in the tyre expands on heating (due to friction with the road surface), the internal pressure increases.
Ammonia, NH3,forms a relatively strong type of intermolecular attraction known as a hydrogen bond, whereas methane, CH4, does not. Explain the relative deviation from ideal behaviour that each gas is likely to show.
NH3 is a polar molecule CH4 is nonpolar. NH3 shows greater deviation than CH4 due to stronger intermolecular attractions, especially at low temperature.
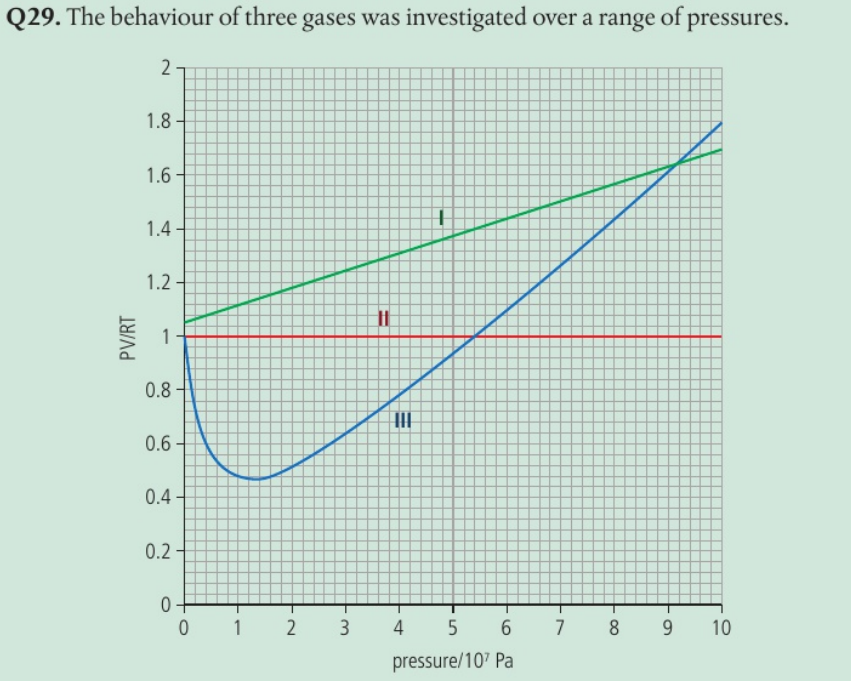
Identify the gas with the strongest intermolecular forces and justify your answer.
III as PV RT < 1 at low pressure
Attractive forces from other particles reduce the speed of the colliding particles and lead to a less energetic collision with the wall.
The pressure is lower than for an ideal gas as it is reduced by intermolecular forces.
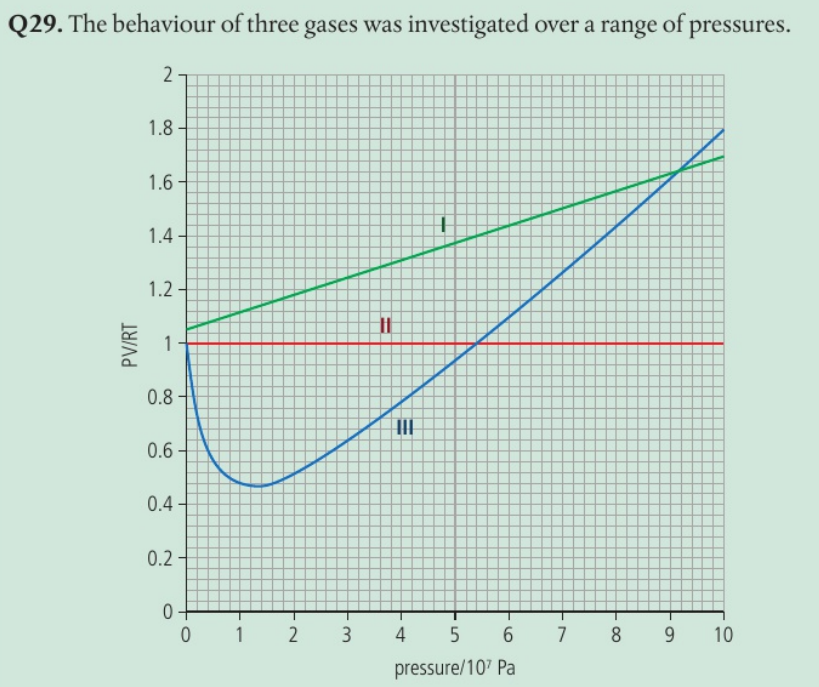
Identify the gas with the largest molecular volume and justify your answer.
III as PV RT >>> 1 as the pressure increases
The pressure is greater than an ideal gas due to the space occupied by the molecules.
The volume of the molecules becomes more significant as the pressure increases.
Explain why helium behaves as an ideal gas over a wide range of pressures, volumes and temperatures.
weak intermolecular/dispersion forces small atomic volume smaller number of electrons
Helium atoms are very small and have a very small atomic volume, so the space taken up by the particles themselves is negligible compared with the volume of the container. This makes the ideal gas assumption that particle volume is negligible much more valid.
Helium also has a small number of electrons, which means its electron cloud is not easily distorted. As a result, the London dispersion (intermolecular) forces are extremely weak. With almost no attraction between particles, helium closely fits the ideal gas assumption that there are no intermolecular forces except during collisions.

c
write the formula of copper II bromide
CuBr2
what is the formula of chromium III sulfate
Cr2(SO4)3
what is the formula of aluminium hydride
AlH3
Explain what happens to the electron configurations of the elements Mg and Br when they react to form the compound magnesium bromide.
The magnesium atom loses its two electrons from the 3s orbital to form Mg2+, with electron configuration [Ne].
Two bromine atoms each gain one electron into their 4p subshell to form Br– , with electron configuration [Kr].
The ions attract each other by electrostatic forces and form a lattice with the formula MgBr2.
Describe the bonding in ammonium nitrate.
Ionic bonding between NH4 + ions and NO3 – ions. Covalent bonding within NH4 + ions and NO3 – ions.
Note the compound does not contain any metal ions.
You are given two white solids and told that only one of them is an ionic compound. Describe three tests you could carry out to determine which is the ionic compound.
Test the melting point: ionic solids have high melting points.
Test the solubility: ionic compounds usually dissolve in water but not in hexane.
Test the conductivity: ionic compounds in aqueous solution are good conductors.
what does it mean by "most ionic"
"Most ionic" refers to a chemical bond or compound with the highest degree of electrostatic attraction and electron transfer, occurring when there is the largest possible difference in electronegativity between elements.

why is b the answer when a has the highest difference in electronegativity
describe the bonding in sodium nitrate
Ionic bonding/electrostatic attraction between Na+ and NO3 – ions. (1)
Covalent bonding between nitrogen and oxygen atoms within the nitrate ion. (1)

b
explain why iron iii oxide can demonstrate brittleness
Applied force moves layers so ions of the same charge are forced to be closer.
Repulsion between layers makes the compound brittle.

bond strength is highest in triple, double or single bonds?
triple
bond length is longest in triple double or single bonds?
single bonds are the longest
d
how to determine if a molecule is polar or nonpolar
A molecule is polar if it has an asymmetrical shape (e.g., bent, trigonal pyramidal) or uneven charge distribution, often caused by lone pairs on the central atom or different surrounding atoms, resulting in a net dipole moment
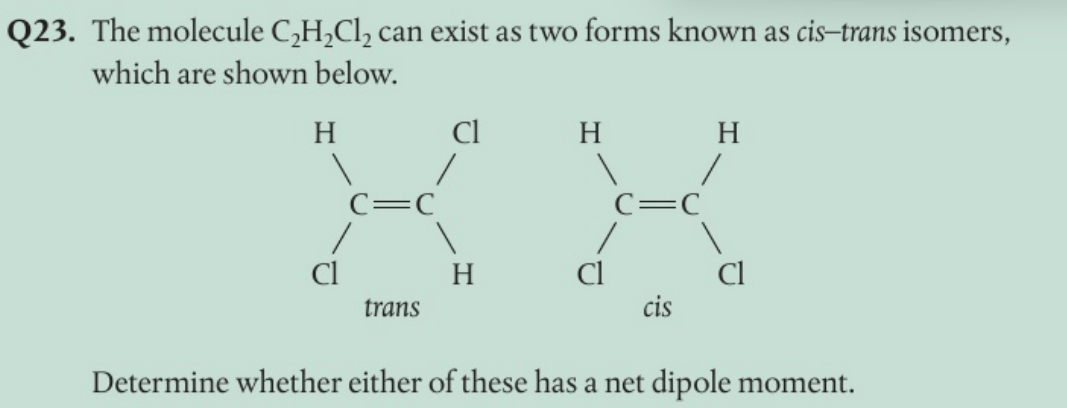
The cis isomer has a net dipole moment as both the electronegative atoms are on the same side of the molecule and so there is an overall dipole moment for the molecule. In the trans isomer the dipoles cancel out.

Similarities: composed of carbon atoms only, both are covalent network structures. Differences:
each carbon is bonded to 4 other carbon atoms in diamond and 3 other carbon atoms in graphite
geometry around carbons is tetrahedral in diamond and trigonal planar in graphite
diamond is continuous C–C structure whereas graphite is distinct layers of carbon atoms (layers are held together by weak intermolecular forces)
graphite has delocalized electrons but diamond does not.

Similarities: strong, high melting points, insoluble in water, non-conductors of electricity, good thermal conductors.
Differences: diamond is stronger and more lustrous; silicon can be doped to be an electrical conductor.
how to tell if a molecule has dipole dipole bonding or not
if it is polar, meaning it has a permanent separation of charge (a positive and negative end) due to unequal electronegativity and an asymmetrical shape
(b)
Compound Y travelled further, meaning it likes the non-polar solvent more. This makes Compound Y less polar (more non-polar).
Compound X stayed back, meaning it prefers the polar stationary phase (usually silica or alumina). This makes Compound X more polar.
what are the two resonance structures of benzene
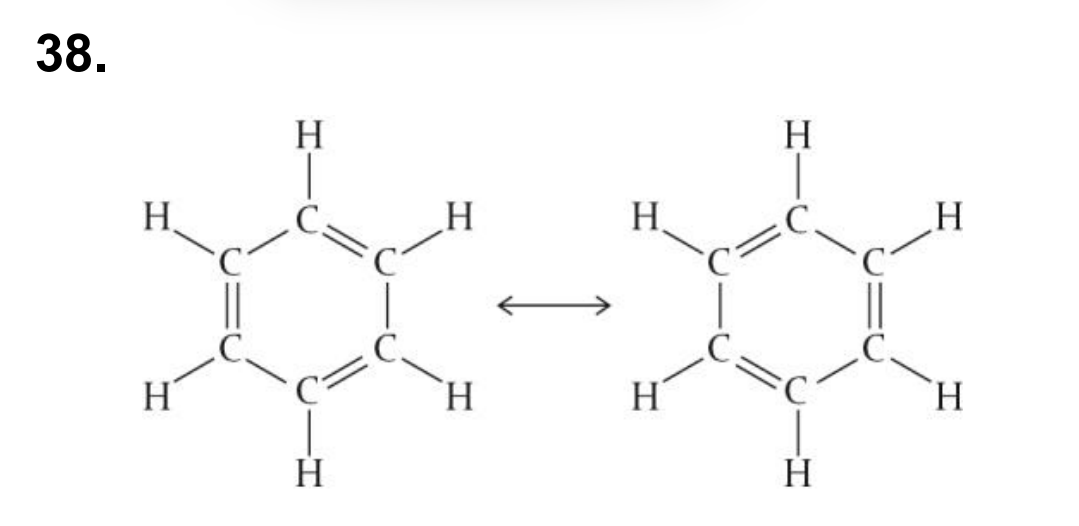

As the delocalized pi electrons in benzene are shared across all bonding positions in the carbon ring, we find the bond lengths are identical and of an intermediate value instead of alternating single and double bond lengths.

Benzene contains six carbon atoms arranged in a hexagonal ring, each also with a single bond to a hydrogen atom.
The bonds between the carbon atoms are identical and have a length and strength that lies in between that of a single and double bond.
This is due to the delocalization of pi electrons which are shared equally across the carbon ring, increasing its energetic stability
The bond enthalpy values for all carbon–carbon bonds in benzene are identical with an intermediate value lying between that of a single bond and a double bond. This is evidence for delocalization - how so?
Delocalization is essentially "electron spreading." By spreading the electron density across all six carbon atoms, benzene achieves a state of lower potential energy and higher stability than a system with alternating bonds.
Identical values prove the symmetry of the molecule (no alternating lengths).
Intermediate values prove that the bonds are "one-and-a-half" bonds—stronger than a single, but weaker than a double.
Benzene is significantly more stable than the theoretical molecule cyclohexa-1,3,5-triene. Explain why benzene undergoes electrophilic substitution reactions rather than addition reactions.
Stability of the Ring: Benzene contains a delocalized pi electron system above and below the plane of the carbon atoms. This delocalization provides the molecule with extra "aromatic stability."
The Cost of Addition: An addition reaction would require breaking this delocalized system to form new sigma (single) bonds with the incoming atoms. This would permanently destroy the aromatic ring and result in a loss of stability.
The Advantage of Substitution: In a substitution reaction, a hydrogen atom is replaced, but the delocalized pi system remains intact. Because the product retains its aromatic stability, this pathway is energetically much more favourable.
what is the bond angle for square planar
90*