Stability
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
What is the difference between shelf life and expiry date?
-expiry date is the end of the shelf life
-shelf life=The amount of time a product is expected to remain usable, effective, and safe when stored under recommended conditions.
Give 5 examples of routes of degradation
-oxidation
-photodegradation
-heat
-solvent degradation
-acid and base catalysis
What can we do if a MP is unstable at room temperature?
-put it in a fridge (2-8 degrees) or freezer (less than -15 degrees) but preferably fridge so that MP doesn’t freeze and also because some MPs e.g. vaccines can degrade by freezing
Increase in temperature can…
-increase the rate of degradation
When is the risk of elevated temperature of the MP the highest?
-when it’s in vehicles for storage and transport. Can be avoided
Give examples of when a drug would hydrolyse in water
-if it contains an amide or ester
What can influence how fast charged species react?
-dielectric constant
How can we reduce the rate of hydrolysis? (3)
-replace water with another solvent e.g. ethanol
-can put in fridge or freezer to decrease the rate of hydrolysis
-turn into solid dosage form as it is not surrounded by solvent molecules or you can make it into a suspension
Why is a suspension good in terms of reducing hydrolysis?
-only the molecules on the outside/surface of the drug particles are vulnerable to degradation and hydrolysis, not the middle of the particle e.g. that’s why if you leave aspirin tablets in moist environments, sometimes it smells like vinegar (due to acetic acid production)
How can we store unstable solid drugs sometimes?
-as freeze-drug powders and then reconstituted (i.e. made up into a solution right before administration instead of months in advance).
What is generally more stable, suspensions or solutions of the same drug?
suspensions
What ions can catalyse hydrolysis?
H3O+ and OH- ions, therefore the pH of the environment is very important
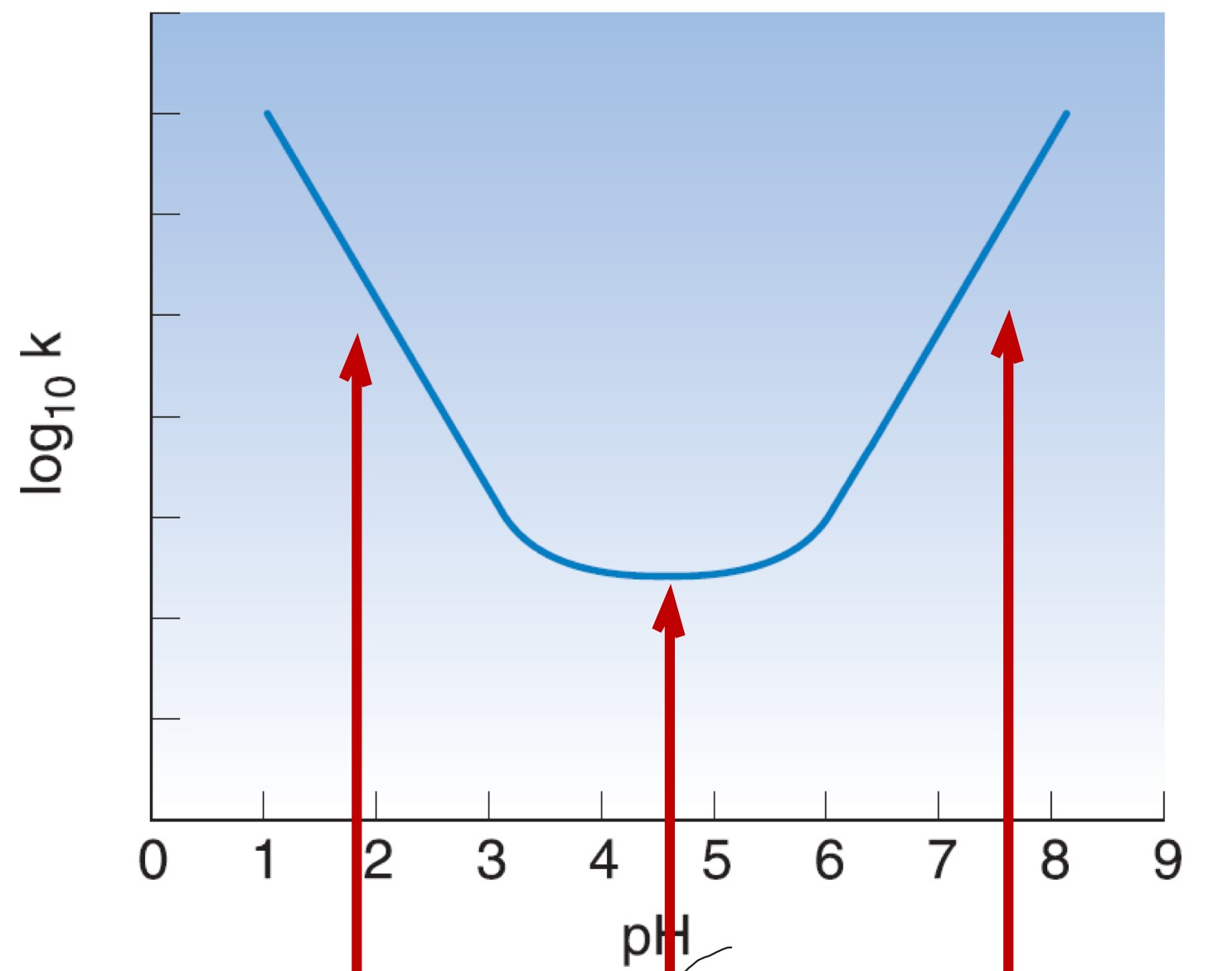
label this. What is the problem in this graph? How can it be solved?
specified acid hydrolysis (hydrolysis catalysed by H+ ions)
-uncatalysed hydrolysis
-specific base hydrolysis
-logk=rate of hydrolysis
-at high and low pH, rate of hydrolysis is high, so degradation rate is high, so shelf-life is shorter. So, add buffering agent to make pH as close to 4.5 as possible
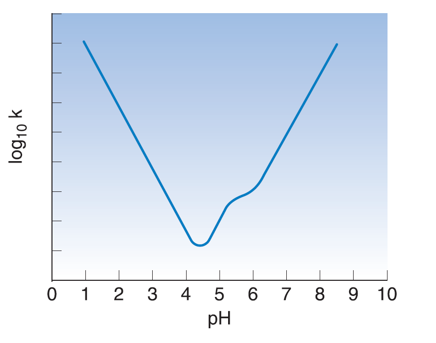
What it this kink called? Why does it happen?
-point of inflection. A point of a curve at which a change in the direction of curvature occurs.
-the pH changes due to relative changes in unionised and ionised forms of drug, so changes reaction rate
What is specified catalysis?
-H3O+ and OH- ions catalysing
What is unspecified catalysis?
-other species other than H3O+ and OH- ions used in degradation
Why can it be bad to use certain buffer ions? What can be used instead?
-can cause degradation
-borate buffer i.e. using borate ions (if it’s compatible with the drug) to not catalyse degradation
Can light and oxygen cause degradation?
yes
What’s the problem for degradation, UV light or visible light?
UV
How can we block UV light?
-tinted glass or container
-use opaque outer container e.g. cardboard
How can we protect our medicinal product from oxidation? (3)
-we can flush the containers with an inert gas(N2, CO2 etc) to replace the oxygen. But, hard to remove all oxygen and best to use in single-use containers
-antioxidants
-trace elements
At what type of pH is oxidation often accelerated at? Why? So, what can we do to tackle this?
-high
-more dissolved oxygen than hydrogen
-therefore, if oxidation is a problem, we can formulate at low pH
What type of ions can catalyse oxidation reactions? Therefore, how can we resolve this problem? (2)
-metal ions e.g. Mg2+
-adding a chelating agent e.g. EDTA to bind to the metal and prevent catalytic activity
-add an antioxidant (reacts with oxygen instead of the drug), which also stops formation of free radicals
Give an example of ascorbic acid
-water soluble
Give 4 different examples of what liquid medications can suffer from and what they lead to
evaporation of volatile components (e.g. peppermint flavour). Leads to poor taste and therefore lack of pt acceptability
sorption of drug to container. Leads to the loss of the drug
shedding of (glass) particles from the glass container. This isn’t dangerous but can change the texture of the medicine in the mouth, therefore, less adherence
Extraction of material(s) from the container into the liquid. This leads to possible toxicity and change in pH
Why is the instability of solutions and suspensions bad? (2)
-solutions may undergo the precipitation of the drug, leading to loss of efficacy and poor appearance
-caking of suspensions, which leads to inaccurate doses being given (overdose or underdose), poor appearance and grittiness.
In emulsions and creams, does cracking mean the chemical structure of the product has changed?
no, but its pharmaceutical quality has changed e.g. harder to apply, not evenly dispersed
What is adsorption in terms of drug particles in a container?
-the particles adhere to the walls of the container
What is absorption in terms of drug particles in a container? Why is it bad? What can be used to stop this?
-absorption of the drug into the container can happen e.g. hydrophobic container and hydrophobic drug molecules
-glass container because it’s hydrophilic
-causes loss of drug from product
Other than the container of the MP, what else can the product sorp onto? What can effect how much sorption occurs?
-non-polar molecules have a high affinity for plastics and rubber (including the lids) (so use glass instead)
-volatile components sorbs to plastic packaging from the solution (so use glass instead)
-pH can affect sorption with ionisable drugs. Unionised form means less polar, hence more sorption
How to test stability of drug in terms of oxidation (preformulation)
heat the solution with and without oxygen flushing
How to test the stability of a drug in terms of hydrolysis (preformulation)
-heat the solution of the drug in water or acid or base
How to test the stability of a drug in terms of light degradation
-shine artificial daylight lamps on the drug
How to test the stability of a drug in terms of temperature
-heat the medicinal product in the solid state at high temperature, alone and/or with excipients
Explain what stress testing is in pharmaceutics. How can arrhenius equation be used? Why is stress testing good?
It involves exposing a drug substance or drug product to extreme environmental conditions to:
Understand its degradation pathways,
Identify degradation products,
Assess the molecule’s intrinsic stability, and
Establish suitable storage conditions and shelf life.
-you can calculate rate of degradation at RT from elevated T measurements
-it’s quicker than waiting years for results in early stages of drug development
Give 4 limitations of stress testing
-the shelf-life prediction is generally poor
-heating can cause moisture levels to decrease in solid particles
-Over-Degradation
Excessive degradation can make it difficult to distinguish relevant impurities from irrelevant ones.
It may complicate the identification of degradation pathways important for shelf life prediction.
-Stress testing uses extreme conditions (heat, light, acid, etc.), which may not accurately mimic actual storage or usage environments. This can lead to degradation products that might not form under normal conditions, possibly skewing the data.
What is temperature cycling?
-repeatedly exposing a drug product or substance to alternating high and low temperatures over a period of time. It's used as a stress test to evaluate the product’s physical and chemical stability, particularly how it tolerates temperature fluctuations that might occur during shipping, storage, or handling.
-accelerates aging and particle growth (suspension), cracking (emulsion) or precipitation (emulsions)
What is important when doing photostability testing? (2)
-make sure you do it with the pure drug first and then with the formulated product
-use the same packaging as will be used when the drug is sold commercially
What is long term testing?
-long term studies under actual storage conditions to be used for the product.
-used to investigate realistic worst case scenarios of temperature and humidity. It’s a much better representation of how long and how much the drug is instable and takes to be instable
Go through the testing protocols for long-term testing (7)
•Use the same packaging for testing as will be used for the product;
•Test at least three batches of the product;
•Store liquid formulations inverted to ensure that the contents can interact with the cap of the container;
•Sample at least every 3 months in the first year, every 6 months in year 2, and annually thereafter until end of proposed shelf life.
•Use batches of at least pilot scale (different batch sizes can have different properties – e.g. viscosity);
•Usually control humidity, unless this does not affect product (e.g. aqueous products in glass containers);
•Aqueous preps in plastic may need to undergo low humidity stress testing, especially for Zone III – because of water loss. Water loss is twice as fast at 30% RH than 60% RH.
Give 9 examples of stability assessments for liquid dosage forms
•Oral solutions, suspensions, emulsions:
–Precipitate formation
–pH
–Viscosity
–Level of microbial contamination.
•Additionally for suspensions:
–Dispersibility
-rheological properties
-size and size distribution of particles
•Additionally for emulsions:
–Phase separation
-mean size and size distribution of dispersed phase.
How do we evaluate the results from long term stability testing? (4)
•Set specifications for the product – degradation must be within acceptable limits (usually leaving > 90% of stated dose);
•Appearance, smell, taste, etc, must also be acceptable;
•Apply statistical tests to ensure that 90% limit is met;
•Test at least 3 batches, calculate shelf lives and take the shortest time!
Why can it be problematic to freeze liquid formulations for storage?
-They need to be defrosted before use, which can cause drug degradation if heat is used to achieve defrosting.
-internal pressure
Why are suspensions generally more stable than solutions?
-In a solution, all the drug molecules are completely surrounded by solvent molecules. If the drug undergoes solvent-mediated degradation, then all the molecules of drug are vulnerable to breakdown. In a suspension, there are particles of drug in a liquid carrier. Only drug molecules at the surface of the particles can react with the liquid carrier – those in the centre of the particles are protected from the liquid phase. Thus, a reduced proportion of the drug molecules are vulnerable to solvent-mediated breakdown in a suspension, and reduced amounts of degradation are expected.
What are specific acid catalysis and specific base catalysis?
-Specific acid and base catalysis refer to the hydrolysis of a drug when catalysed by H3O+ (acid) or HO- (base).
Sketch how the rate of degradation for a drug is likely to vary with pH if the drug is ionisable.
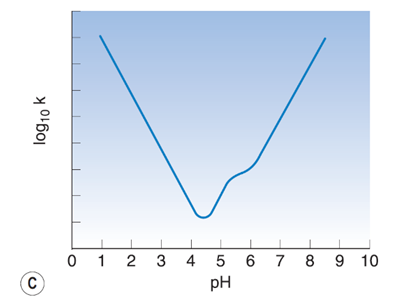
Why is the choice of buffer important in preparing a liquid formulation? (2)
- First, we need to make sure that the pH of the solution is appropriate to minimize degradation, and to do this we must choose the right buffer.
-sometimes the ions / molecules in the buffer can react with the drug and cause degradation, and so we need to select a buffer where this does not occur.
How would you package a liquid formulation which is sensitive to light and oxygen, if the patient is to take only three doses over the course of one day?
-you could use a tinted glass container (to protect from light) and flush it with an inert gas such as N2 or Ar prior to sealing (to remove as much oxygen as possible). Although some oxygen will get in when the container is opened, this should not be a big problem as it will only be opened three times.
Explain how an anti-oxidant helps to improve the stability of a drug. Give in your answer an example of an oil-soluble anti-oxidant.
-Antioxidants react with O2, removing it from the formulation, and also terminate free radical interactions. α-tocopherol is an example of an oil soluble antioxidant.
three forms of physical instability which may arise with a liquid formulation.
e.g. sorption, shedding of particles
What is caking?
-when the particles in a suspension sink and results in a very dense sediment with minimal liquid entrapment, which is very hard to redisperse upon shaking. As a result, if caking arises in a pharmaceutical suspension then the drug will mostly be stuck at the base of the container, and when the patient removes a dose they may not get any of the active ingredient: certainly, there will be very high dose variability with a caked suspension.
What is sorption?
-when the drug in a liquid formulation becomes attached to the container. This can comprise either adsorption (where the drug molecules or particles become stuck to the internal surface of the container) or absorption (where the drug is taken up inside the container walls).
Explain the differences between the four global climactic zones. Why is it important to study product stability in all the zones where the product will be sold?
-the four zones differ in their average temperatures and relative humidities. It is important to study product stability in all the zones where a product will be sold in order to ensure that it is stable in that set of conditions: temperature and relative humidity (RH) will affect degradation rates and processes.
Why do we test multiple batches of a product during long-term stability testing?
-Because there will be batch to batch variability, and not all batches will be identical! We thus need to test several batches to make sure we fully understand this variability and have a good overall understanding of how the formulation will behave.