Biology 1610 Exam 2
1/224
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
225 Terms
What is Kinetic Energy?
Associated with the relative motion of objects
What is Potential Energy?
Refers to an object not presently moving; it is the energy that matter possesses because of its location or structure
Which type of energy does water behind a dam have?
It has potential energy.
Which type of energy does a mole of glucose have?
It also has potential energy, but more specifically it has chemical energy
What type of energy do the diffusion of ions through an ion channel have?
They have kinetic energy.
What is the 1st law of thermodynamics?
Energy can be transferred and transformed, but it cannot be created or destroyed. The amount of energy in universe is constant.
What is the 2nd law of thermodynamics?
Every energy transfer or transformation increases the entropy of the universe. Some call this the “you always lose rule”.
What do the laws of thermodynamics mean for energy in the universe?
During every energy transfer or transformation some energy is converted to thermal energy and released as heat, becoming unavailable to do work. A consequence of the loss of usable energy as heat to the surroundings is that each energy transfer or transformation makes the universe more disordered, increasing entropy of the universe.
What is free energy?
The portion of a system’s energy that can perform work when temperature and pressure are uniform throughout the system, as in a living cell.
What is free energy symbolized by?
The letter G
For an exergonic reaction is ΔG negative or positive?
Proceeds with a net release of free energy. Because the chemical mixture loses free energy, ΔG is negative.
Is cellular respiration an endergonic or an exergonic reaction?
An exergonic reaction
Is photosynthesis endergonic or exergonic?
An endergonic reaction
What is the energy sources that drives photosynthesis?
Plants get the required energy to make a mole of glucose from the environment by capturing light energy from the sun and converting its energy into chemical energy.
What is the difference between catabolic and anabolic pathways?
Catabolic pathways are the cellular process of breaking down large molecules into smaller ones, releasing energy. Anabolic pathways build larger molecules from smaller ones, requiring energy.
What is energy coupling?
The use of energy released from an exergonic reaction to drive an endergonic reaction.
How does energy coupling drive endergonic reactions in cells?
Hydrolysis of ATP to ADP and Pi is an exergonic reaction that is used to drive endergonic reactions. Or the protons diffusing across the gradient is an exergonic process used to drive the endergonic process of creating ATP.
When the terminal phosphate bond of ATP is broken, a molecule of inorganic phosphate Pi is formed, and energy is ______. Is this reaction endergonic or exergonic?
Released, exergonic
What is a catalyst?
A chemical agent that selectively increases the rate of a reaction without being consumed by the reaction.
What is activation energy (EA)?
The amount of energy that reactants must absorb before a chemical reaction will start; also called free energy of activation.
What effect does an enzyme have on EA?
Catalyzes a reaction by lowering the EA barrier.
How is ^G affected by the enzyme?
Unaffected by the enzyme. The enzyme cannot change the ^G for a reaction; it cannot make an endergonic reaction exergonic.
Define enzyme:
A macromolecule serving as a catalyst, a chemical agent that increases the rate of a reaction without being consumed by the reaction. Most are proteins.
Define substrate:
The reactant on which an enzyme works.
Define Active Site:
Typically, a pocket or groove on the surface of an enzyme where the substrate binds and catalysis occurs.
Define Products:
A material resulting from a chemical reaction.
What is meant by induced fit?
The slight change in the shape of the active site of an enzyme so that it binds more snuggly to the substrate.
Explain how protein structure is involved in enzyme specificity?
Enzymes are proteins, and proteins are macromolecules with unique three-dimensional configurations. The specificity of an enzyme results from its shape, which is a consequence of its amino acid sequence. The specificity of an enzyme is attributed to a compatible fit between the shape of its active site and the shape of the substrate.
What is a cofactor?
Any nonprotein molecule that is required for the proper functioning of an enzyme.
Can be permanently bound to the active site or may bind loosely and reversible, along with the substrate, during catalysis.
Examples of cofactors;
Some enzymes are inorganic such as the metal atoms zinc, iron, and copper in ionic form.
What is a coenzyme?
An organic molecule serving as a cofactor.
Examples of a coenzyme:
Most vitamins are important in nutrition because they act as coenzymes or raw materials from which coenzymes are made.
Name a human enzyme that functions well in pH 2.
Pepsin
Where is pepsin found?
A digestive enzyme found in the human stomach.
Why can extremes of pH or very high temperatures affect enzyme activity?
The 3D structures of proteins are sensitive to their environments. As a consequence, each enzyme works better under some conditions than other conditions, because these optimal conditions favor the most active shape for their enzyme molecule.
Competitive inhibitors:
Substances that reduce the activity of an enzyme by entering the active site in place of the substrate, whose structure it mimics.
Noncompetitive inhibitors:
Substances that reduce the activity of an enzyme by binding to a location away from the active site. This change the enzyme’s shape in such a way that the active site becomes much less effective at catalyzing the conversion of substrate to product.
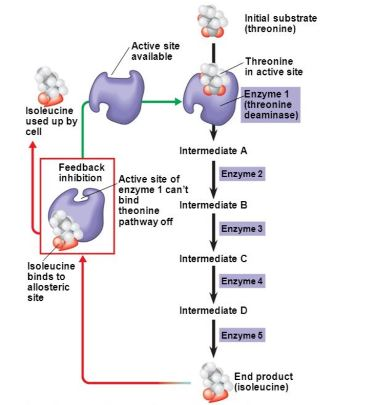
Describe feedback inhibition as a mechanism to control metabolic pathways?
The end product of a metabolic pathway often serves to inhibit the production of more of its own molecules. This preserves raw materials for biosynthesis.
Fermentation
A partial degradation of sugars or other organic fuel that occurs without the use of oxygen.
Aerobic Respiration:
Consumes oxygen as a reactant along with the organic fuel.
What are the products of glucose oxidation?
The breakdown of glucose in cellular respiration yields carbon dioxide, water, and energy.
What is oxidation?
In a redox reaction the loss of electrons from one substance
What is reduction?
The addition of electrons to another substance.
When compounds lose electrons, they _______ energy; when compounds gain electrons, they _______ energy.
Lose; gain
In cellular respiration, electrons are not transferred directly from glucose to oxygen. Following the movement of hydrogens allows you to follow the flow of electrons. What electron carrier is hydrogen transferred to first?
NAD+
Describe what happens when NAD+ is reduced.
The enzymatic transfer of 2 electrons and 1 proton (H+) from an organic molecule in food to NAD+ reduced the NAD+ to NADH; the second proton (H+) is released.
NAD+ is the ______ form, and NADH is the _____ form.
Oxidized (low energy) form, Reduced (high energy) form
What is the function of the electron transport chain in cellular respiration?
Shuttles electrons down a series of redox reactions that release energy used to make a proton gradient that is ultimately used to generate ATP.
Which strongly electronegative atom, pulling electrons down the electron transport chain, in the final electron acceptor?
Oxygen
Explain how the electron transport chain is utilized in oxidative phosphorylation.
This mode of ATP synthesis is powered by the redox reactions of the electron-transport chain. The energy released in each step in the electron transport chain provides the energy to maintain the chemiosmotic gradient that powers the ATP synthase complex.
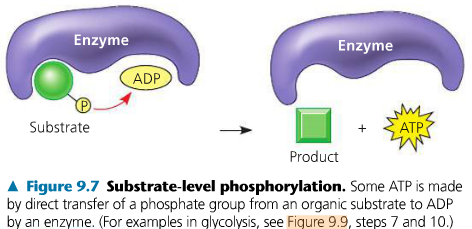
The second form of phosphorylation is substrate level. Label the following figure to show the direct transfer of a phosphate from an organic substrate to ADP to form ATP. Which stages use SLP?
Glycolysis and Citric acid cycle
Where does glycolysis occur?
Cytoplasm
Where does pyruvate oxidation occur?
Mitochondria.
Where does the Citric Acid Cycle occur?
In the matrix of the mitochondria
Where does oxidative phosphorylation occur?
Mitochondria..
What is the meaning of glycolysis?
Sugar-splitting (cutting)
What occurs during glycolysis?
It’s a series of reactions that ultimately splits glucose into pyruvate. It occurs in almost all living cells, serving as the starting point for fermentation or cellular respiration
Is oxygen required for glycolysis?
No
The starting product of glycolysis is the six-carbon sugar ______ and the ending products are two ______-carbon molecules of ________.
Glucose, three, pyruvate
What is energy investment in glycolysis?
Invest 2 ATP to phosphorylate glucose.
What is energy payoff in glycolysis?
Oxidize G3P to create NADH and generate 4 ATP from substrate level phosphorylation
What are the inputs of glycolysis?
Input is 2 ATP, Glucose, 2 NAD+
What are the outputs of glycolysis?
4 ATP, 2 NADH, 2 Pyruvate. Used 2 ATP, gained 4 ATP, net gain of 2 ATP gained in glycolysis
Glycolysis is thought to have evolved very early in the evolution of life on Earth. Provide three pieces of evidence that justify this hypothesis.
The cytosolic location of glycolysis implies great antiquity.
The pathway does not require any of the membrane-bound organelles that evolved approximately 1 billion years after the first prokaryotic cell.
The most widespread metabolic pathway among Earth’s organisms.
Explain what has happened to the six carbons found in the original glucose molecule
Two molecules are released from the conversion of pyruvate to acetyl CoA and four molecules are released from the Citric Acid Cycle
How many NADHS are formed in the citric acid cycle: (after Acetyl-CoA is produced)
3 from each Acetyl-CoA
How many total carbons are lost as Acetyl-CoA is oxidized? (Citric Acid Cycle)
2
The carbons are lost in which molecule? (Citric Acid Cycle)
CO2
How many FADH2 are formed? (Citric Acid Cycle)
1/Acetyl CoA
How many ATPs are formed? (Citric Acid Cycle)
1/Acetyl CoA
How many times does the citric acid cycle occur for each molecule of glucose?
Two
Note that little ATP has been produced by the end of CAC. Where is most of the energy at this point in cellular respiration?
NADH and FADH2
Explain why oxygen is considered the ultimate electron acceptor. What is it’s role in cellular respiration?
It’s extremely electronegative. It is the final electron acceptor.
Oxygen stabilizes the electrons by combing with two hydrogen ions to form what compound?
H2O
What is the role of the electron transport chain in forming the H+ gradient across the inner mitochondrial membrane?
Certain members of the electron transport chain accept and release protons (H + ) along with electrons. (The aqueous solutions inside and surrounding the cell are a ready source of H + .) At certain steps along the chain, electron transfers cause H+ to be taken up and released into the surrounding solution. In eukaryotic cells, the electron carriers are spatially arranged in the inner mitochondrial membrane in such a way that H+ is accepted from the mitochondrial matrix and deposited in the intermembrane space. The H+ gradient that results is referred to as a proton-motive force, emphasizing the capacity of the gradient to perform work. The force drives H + back across the membrane through the H+ channels provided by ATP synthases.
What is the electron acceptor in fermentation?
NAD+
What must be regenerated for fermentation to continue producing 2ATP per glucose?
NAD+
What three organic macromolecules are often utilized to make ATP by cellular respiration?
Proteins, carbohydrates, and fats are utilized to make ATP by cellular respiration.
What are autotrophs?
Self feeders; they sustain themselves without eating anything derived from other living beings. They produce their own organic molecules from CO2 and other inorganic raw materials obtained from the environment.
Are autotrophs or heterotrophs the produces of the ecosystem?
Because they are ultimate sources of organic compounds for all nonautotrophic organisms, autotrophs are the producers of the biosphere.
What are some examples of autotrophs?
The plants, multicellular alga, unicellular protists, cyanobacteria, and purple sulfur bacteria.
What are heterotrophs?
Unable to make their own food; they live on compounds produced by other organisms.
Which are the consumers of the ecosystem?
Heterotrophs
What are some examples of heterotrophs?
Animals, decomposers, most fungi, and many prokaryotes
Where do the light reactions take place in chloroplasts?
The thylakoid membrane
Where do light reactions take place in the Calvin Cycle?
In the stroma
Explain what occurs in the light reactions stage of photosynthesis.
Water is split providing a source of electrons and protons (hydrogen ions, H+) and giving off O2 as a by-product. Light absorbed by chlorophyll drives a transfer of the electrons and hydrogen ions from water to an acceptor called NADP+, where they are temporarily stored. The light reactions use solar power to reduce NADP+ to NADPH by adding a pair of electrons along with an H+. The light reactions also generate ATP, using chemiosmosis to power the addition of a phosphate group to ADP, a process called photophosphorylation.
Explain the Calvin Cycle.
The cycle begins by incorporating CO2 from the air into organic molecules already present in the chloroplast. This initial incorporation of carbon into organic compounds is known as carbon fixation. The Calvin Cycle then reduces the fixed carbon to carbohydrate by the addition of electrons. The reducing power is provided by NADPH, which acquired its cargo of electrons in the light reactions. To convert CO2 to carbohydrate, the Calvin Cycle also requires chemical energy in the form of ATP, which is also generated by the light reactions.
What does the light reaction provide to the Calvin Cycle?
They provide ATP and NADPH to the Calvin Cycle.
What does the Calvin Cycle provide to the light reactions?
Provides NADP+ and ADP back to the light cycle to be reused.
What is the relationship between the light reactions and the Calvin Cycle?
The light reactions convert solar energy to the chemical energy of ATP and NADPH
Explain the relationship between wavelength and energy
Light is a form of energy known as electromagnetic energy, also called electromagnetic radiation. Electromagnetic energy travels in rhythmic waves analogous to those created by dropping a pebble into a pond. The distance between the crests of electromagnetic waves is called the wavelength. The amount of energy is inversely related to the wavelength of light: the shorter the wavelength, the greater the energy of each photon of light. Alternatively, the longer the wavelength of light, the lower the amount of energy of each photon.
Describe how he determined an action spectrum long before the invention of a spectrophotometer
In 1883, Theodor W. Engelmann illuminated a filamentous alga with light that had been passed through a prism, exposing different segments of the alga to different wavelengths, He used aerobic bacteria, which concentrate near an oxygen source, to determine which segments of the alga were releasing the most O2 and thus photosynthesizing most. Bacteria congregated in greatest numbers around the parts of the alga illuminated with violet-blue or red light.
Explain the correlation between an absorption spectrum and an action spectrum.
An absorption spectrum is the range of a pigment’s ability to absorb various wavelengths of light; also a graph of such a range. An action spectrum is a graph that profiles the relative effectiveness of different wavelengths of radiation in driving photosynthesis.
Photosystem II (PSII) has at its reaction center a special pair of chlorophyll a molecules called PS80. What is the explanation for this name?
Because these molecules are best at absorbing light energy at 680 nm. Nearly identical chlorophyll a molecules are found within the reaction center of PS I, however, these molecules of chlorophyll A absorb light energy best at 700 nm. Because of their association with different proteins in the thylakoid membrane that affect the electron distribution in the two pigments, this will account for the slight differences in their light-absorbing properties.
What is the name of the chlorophyll A at the reaction center of PS II? P680+ may be the strongest biology oxidizing agent. Why is this necessary? What molecule is it oxidizing?
P680; it is pulling electrons from the oxygen atom in water, which does not want to be oxidized. It obtains electrons from the oxygen atom in a water molecule, so it must have a greater affinity for electrons that oxygen has.
What is the source of O2 in the atmosphere?
Splitting of water
As electrons fall from photosystem II to photosystem I, the cytochrome complex uses the energy to pump ions. This builds a proton gradient that is used in chemiosmosis to produce what molecule?
In photosystem I, NADP+ reductase catalyzes the transfer of the excited electron and H+ to NADP+ to form
NADPH
Photophosphorylation is the most similar to what process in what cellular respiration?
Oxidative phosphorylation