extended and delayed release oral formulations
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
what formulation types are classified as extended release? (2)
sustained release
controlled release
advantages of extended release medications? (4)
less fluctuation in blood drug level
reduced SEs
reduction in dosing → enhanced convenience + compliance
maintaining therapeutic activity for extended time
disadvantages of extended-release dosage forms (2)
loss of flexibility in adjusting the drug dose + dosage regimen
risk of dose dumping (sudden and total drug release) due to technology failure
what should be considered before developing an extended release dosage form? (2)
suitability of the drug to be formulated into XR
solubility + permeability
suitability of an XR formulation in treating a medical condition
some conditions only need prn dosing - XR would not be ideal
class I XR formulation
most suitable
are high sol + high perm. drugs
drug release from dosage forms can be the rate-limiting step, altered by the dosage form design
class II XR formulation
low sol. high perm. drugs
rate limiting step is on drug dissolution itself, so already has inherent sustained release behaviour
dissolves v slowly, and as long as the resident time of the tab in the intestine is long enough, you will pretty much get all of the drug to be released
may or may not benefit from XR formulation
class III XR formulation
high sol, low perm. drugs
not suitable as absorption is already rate-limited
as the drug cannot permeate well, no matter how much drug is released and the rate at which the drug is released (fast/slow) will not change the fact that it will not absorb well
class IV XR formulation
low sol. and low perm.
most challenging to formulate
due to its low sol and perm, it is already challenging enough to give this drug as an oral formulation
unless it is being used to treat a local GI tract condition → i.e. don’t want the drug to absorb, but to act locally on the surface of the GI
to be a successful XR product… (4)
drug released at a predetermined rate
dissolved in GI fluids
maintain sufficient GI residence time
absorbed at a rate that replaces the amt of drug metabolised + excreted
characteristics of drug candidates for XR products (5)
used for chronic Tx, rather than acute
acute req. more dose adjustments
absorbed uniformly across GI tract
i.e. BCS class I drugs
high potency of drug (admin in small doses)
allows for practical compression of a single daily dose into a manageable tab size
relatively short half life (4-6hrs) with no active metabolites
good TI
in event of dose dumping, ensures it will not lead to significant SEs or emergency intervention
oral XR control technology (5)
chemical rxn/ix between medication + site-specific biologic fluids
barrier coatings → modify drug dissolution and access of biological fluids to the drug
controlling drug diffusion rates from dosage form
erosion control
drug transport control (e.g. osmotic pump)
what do matrix formulations contain?
certain types of insoluble/hydrophilic polymers, which are incorporated throughout the dosage form matrix
polymer will form an internal structural network, with spots of drug captured/embedded in within
as the matrix is insoluble → will be an intact unit throughout the process of transit thru the GI
what are the 4 processes that facilitate the rate and extent of drug release in matrix formulations?
liquid surrounding the dosage form penetrates the release unit
results in hydration of the tablet (swelling/dissolution) from the outside in
diffusion of water into the device
dissolution of the drug to form a solution
diffusion of the dissolved drug out of the device
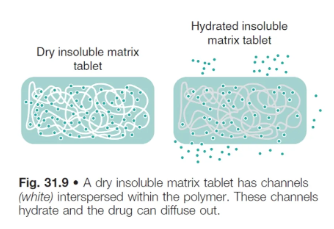
what type of polymer is used in an insoluble polymer matrix?
an inert, water-insoluble polymer, such as:
polyvinyl chloride
polyethylene
polyvinyl acetate
polymethacrylate
what is the rate of drug release from insoluble polymer matrices controlled by? (3)
pore size
no. of pores
tortuosity (twisting path) of the matrix
pore forming agents can be added to increase tortuosity and facilitate drug release
hydrophilic matrix systems can also be referred as…
swellable soluble matrices
what kind of extended release is hydrophilic matrix systems used for?
sustained release
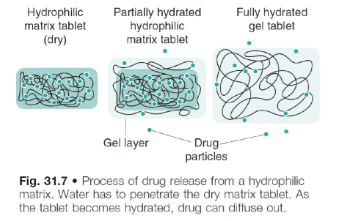
how are hydrophilic matrix systems formulated?
the drug is mixed with a water-swellable, hydrophilic polymer and compressed into a tablet
polymer is in powder/granule form and tablet is manufactured as per normal using the dry granulation process
the final tablet has drug material interspersed between polymer particles
process of drug release from hydrophilic matrix systems
polymer material in tablet swells on fluid contact, producing a gel matrix
gel allows drug release via either:
gel dissolving with the drug trapped within in
gel eroding, which releases/dissolves drug particles trapped within in
what affects the drug release rate from a hydrophilic matrix system?
the rate at which water can diffuse through the tablet, and later through the hydrated gel
the rate of hydration is affected by the gel’s structure
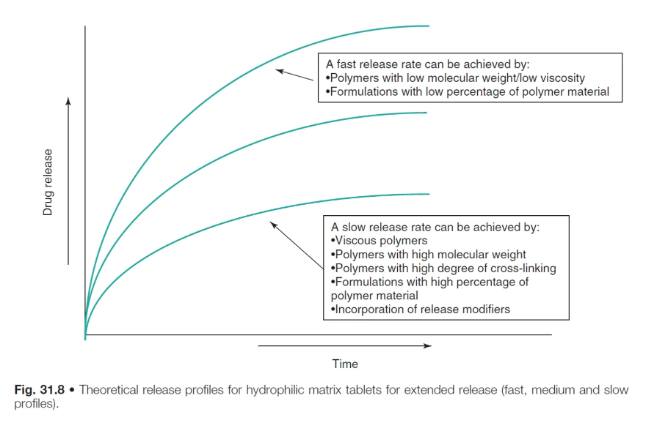
how does polymer type and concentration affect drug release in hydrophilic matrix systems?
the spaces between the matrix forms a tortuous pathway through which water and drug may diffuse
the tortuosity of this pathway is therefore impt for drug release
polymers with low MW/viscosity → fast release rate
polymers with high MW/viscostiy, high degree of cross-linking and high % of polymer material → slow release rate
what is the drug release rate controlled by in erosion controlled release systems?
the erosion of the matrix in which the drug is dispersed
i.e. a surface erosion → continuous reduction in tablet weight during the course of the release process
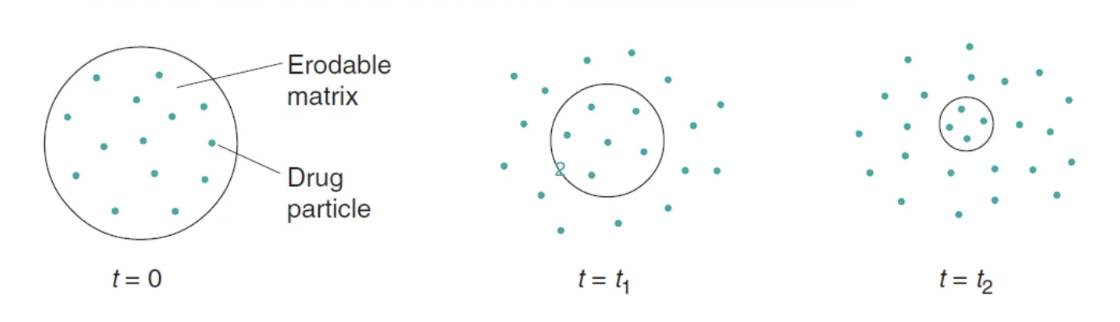
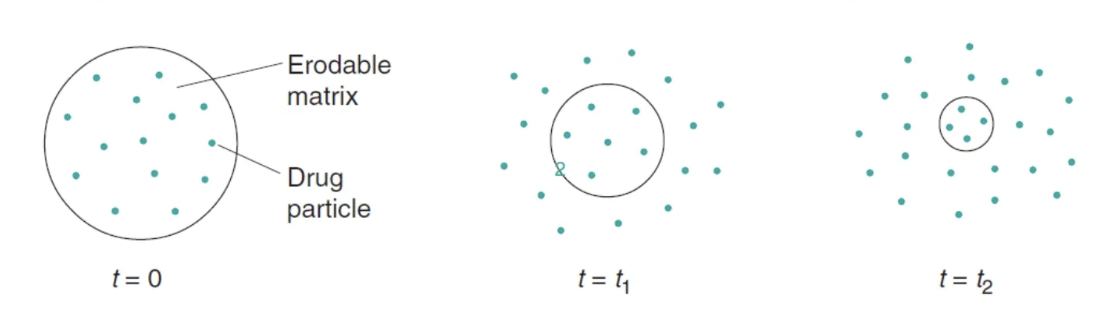
process of drug release from erosion controlled release systems
polymer becomes hydrated after ingestion
drug is then exposed to GI fluids and mixed with (if drug is dissolved in matrix), or dissolved in (if drug is suspended in matrix) the fluid
a gel layer forms around the tablet’s surface and the gel layer thickness increases as water permeates further into the tab
outer gel layer erodes from the tablet core once is fully hydrated → thus rate of drug release is controlled by diffusion and tab erosion
what order is the release rate of a drug from erosion controlled release systems?
zero-order for a significant part of the total release time
what substances is the eroding matrix formed from?
lipids/waxes
drug is dispersed within
hydrophilic cellulose polymers
which gel on contact with water
drug can be either dissolved or dispersed within
hydroxypropyl methylcellulose (HPMC)
what does the polymer need to do for a successful hydrophilic matrix system?
the polymer must form a gelatinous layer rapidly enough to protect the inner core of the tablet from disintegrating too rapidly after ingestion
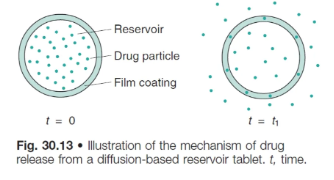
what is the drug release rate controlled by in membrane controlled systems?
it is controlled by the thickness and porosity of the membrane through which the drug must diffuse (rather diffusing through the whole matrix) and the solubility of the drug in the GI fluids
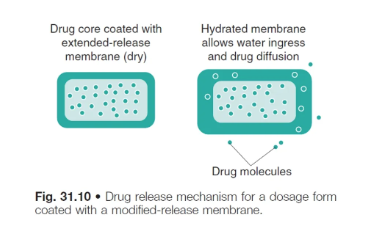
process of drug release from membrane-controlled systems
the tablet is formulated with 2 distinct layers → polymer membrane + drug core compartment
on exposure to fluids, water diffuses into the system to form a continuous phase through which drug diffusion and release can occur
as long as a constant drug conc. gradient is maintained, the release rate will be constant (i.e. zero order)
high MW poylmer membrane
film is normally formed from a high MW polymer → approx 5-20 nanometres thick
what can the membrane porosity and pore tortuosity can be affected by?
adding water sol. components to the membr
in the presence of water, the water sol polymers will dissolve, forming tunnels/channels, which then allows water to enter the core and diffuse drug out
what is the difference between an osmotic pump system and the ‘classic’ membrane controlled system?
only 1 diffusion process is required
in this case, water is able to only able to diffuse in, via a semi-permeable membrane mechanism
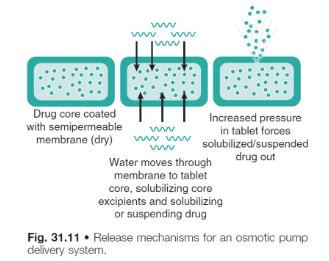
process of drug release from osmotic pump systems
osmotic transport of liquid into the unit
the tablet core is coated with a semi-permeable membrane that allows only water to pass into the core
drug dissolves once water diffuses into the core
dissolution of drug increases the internal hydrostatic force
drug solution/suspension is then forced out of the tablet via a laser drilled orifice due to increasing hydrostatic pressure inside the tablet
drug does NOT diffuse out of the semi-permeable membr as it is NOT permeable to the drug
what do osmotic pump systems require exposure to in order to build up internal osmotic pressure?
exposure to sufficient fluid → which depends on the fluid levels in the GI
what is the drug release rate controlled by in osmotic drug systems?
the rate at which water can pass thru the membr. and how quickly the drug soln/suspension can pass out of the hole
the rate of water flow in is driven by a difference in osmotic pressure between the inside and the outside of the tab
changing the viscosity of the soln/susp formed inside the system also alters the rate at which the drug is forced out
orifice size in osmotic drug systems
needs to be small enough to prevent diffusion, but large enough to minimise hydrostatic pressure
600 micrometer to 1mm diameter
what are gastro-retention drugs used for?
retains dosage form for longer in the stomach to maximise drug absorption in the upper GIT:
drugs for local action in the stomach (e.g. H. pylori infection)
drugs w narrow absorption window in small intestine
drugs degraded in the colon
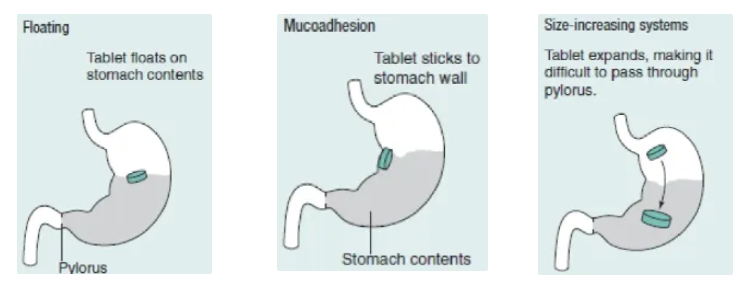
what are the 3 gastro-retention drug delivery systems?
floating
tab floats on stomach contents due to being less dense, thus avoiding gastric emptying
mucoadhesion
tab sticks to the stomach wall to be retained in the stomach
size increasing
tab expands in the stomach, making it harder to pass through the pyloric sphincter so it is retained in the stomach
what are the advantages of delayed release formulations? (4)
protecting the active substance against degradation due to low pH
targeting the active substance to a specific segment of the GI tract for local tx or better absorption
improve bioavail of protein/peptide drugs
targeting active substance release at specific time points
what is coated formulation?
when a modified-release coating is applied to a dosage form
drug is contained in the core and is released/dissolved through the modified release coat
what are the 2 different types of film coating?
immediate release film coatings
modified release film coatings
delayed release (enteric coated)
extended release coatings
enteric coating/gastro resistant formulations are only soluble in pH…
pH >5-6, depending on the polymer
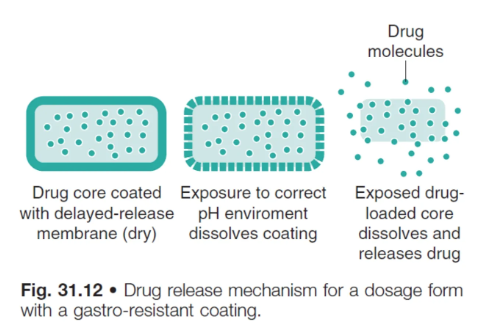
advantages of gastro-resistant coatings (2)
protect stomach from the drug (gastric irritants)
protect acid-sensitive drugs from being degraded in stomach environment
what is the drug release rate of gastro-resistant coatings controlled by?
its exposure to the correct pH
colonic drug delivery formulation
can be achieved by using pH-responsive polymers (dissolve around pH 7) to target the colon
or the use of gut bacteria as a trigger for drug release
what is the highest pH in the GIT?
generally at the ileocaecal junction, just before the colon
the pH is around 7
why is targeting the colon difficult?
the dosage form may be in the region of the highest pH (at the ileocaecal junction) for only a short time, and the target pH (often 7) may not be reached
hence this can lead to dosage form failure → does not disintegrate and is passed intact in stools, thus no drug is released
how is gut bacteria colonic drug delivery formulated?
a coating is prepared from material that is insoluble to GI fluids
but will also contain a component that can only be digested by colonic bacteria, and not by pancreatic enzymes
what component is used in the coating for gut bacteria colonic drug delivery?
a polysaccharide known as ‘resistant starch’ which can only be broken down by bacterial enzymes in the colon
the starch component is digested and dissolved in the colon, leaving pores through which the drug can be released
single unit delayed release dosage form
aka monolithic dosage forms
conventionally manufactured → via compaction + film coating
as they do not disintegrate in the stomach, the tablet could become trapped in the stomach for a long time, esp in the presence of food
this can prevent drugs targeted to the small/large intestine from reaching their site of action
multiple-unit delayed release dosage form
e.g. pellets/granules filled into a hard capsule shell
advantages of multiple-unit delayed release dosage form
more reproducible gastric emptying → because of its small size, can pass thru the sphincter and into the SI more readily
reduced risk of dose dumping
if a tab coating fails, then the whole dose can be dumped
however with a pellet formulation, the disruption of one pellet coating may release only a small fraction of the drug dose
benefits of coating of multi-particulates
capitalising on small size (0.5-2mm)
particles <2mm can pass thru the constricted pyloric sphincter even during the digestion phase
can distribute themselves more readily throughout the GIT
minimising irritant effects
small size reduces likelihood of being lodged in the GIT which can cause localised release of the drug and therefore mucosal damage (if the drug has irritant properties)
drug conc. is also spread out over a larger no. of discrete particles
reducing consequences of imperfect coatings
dose dumping due to imperfections in the coating such as pores can compromise performance of MR dosage forms
but with multi-particulates → risk is reduced as an imperfect coating on a few particles is unlikely to cause harm or lack benefit to the pt