topic 5: energy changes
1/28
Earn XP
Description and Tags
complete! aqa triple higher :(
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
29 Terms
what is an exothermic reaction? provide 3 examples and 2 everyday uses
a reaction that transfers energy to the surroundings so the temperature of the surroundings increases
e.g. combustion, neutralisation, some oxidation reactions
often used in self heating cans and hand warmers
what is an endothermic reaction? provide 2 examples and 1 everyday use
a reaction that takes in energy from the surroundings so the temperature of the surroundings decreases
e.g. thermal decompositions, the reaction between citric acid and sodium hydrogencarbonate
often used in sports injury packs
can energy be created or destroyed?
no! only transferred
(required) describe a practical used to investigate the variables that affect temperature changes
measure a 10cm3 of dilute HCl and pour it into the polystyrene cup
stand the cup inside the beaker, making it more stable
use the thermometer to measure the temperature of the acid and record in a table
rmeasure 5 cm3 of NaOH solution and pour it into the polystyrene cup
fit the lid and gently stir the solution with the thermometer through the hole
look carefully at the temperature rise on the thermometer - when the reading on the thermometer stops changing, record the highest temperature reached in the table
repeat the experiment, adding further 5 cm3 amounts of NaOH to the cup each time, recording your temperature reading in the results table
repeat until a maximum of 40cm3 of NaOH has been added
wash out all the equipment and repeat the experiment for your second trial, to calculate a mean

what things need to occur for a reaction to take place?
particles collide successfully
particles collide with sufficient energy
what is activation energy?
the minimum energy required for particles to react
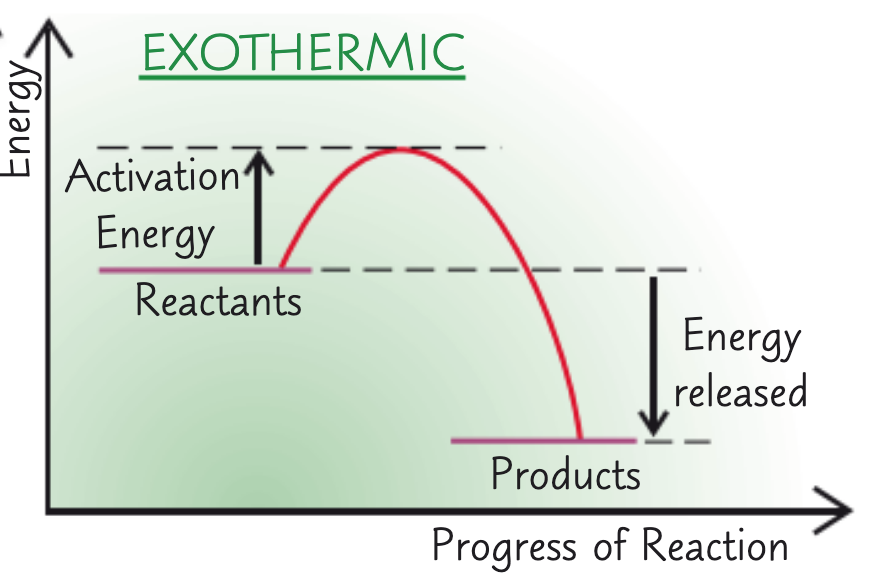
what does the reaction profile for an exothermic reaction look like?
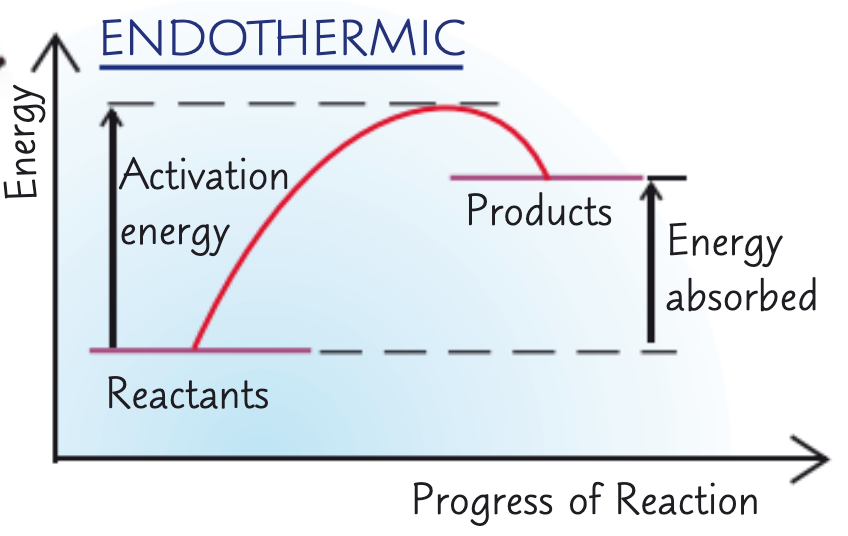
what does the reaction profile for an endothermic reaction look like?
what is on the x and y axis of a reaction profile?
energy, progress of reaction (respectively)
in a reaction profile, what does the arrow on the left hand side represent?
activation energy
in a reaction profile, what does the arrow on the right hand side represent?
exothermic - energy released
endothermic - energy absorbed
in a reaction profile, what do the lines represent?
reactants, products (respectively)
what happens to bonds in a chemical reaction?
energy must be supplied to break bonds in the reactants - bond breaking is an endothermic process
energy is released when bonds in the products are formed - bond forming is an exothermic process
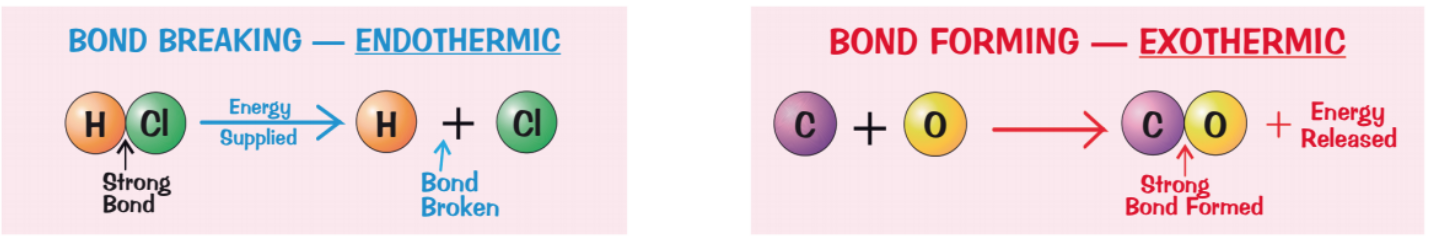
in an exothermic reaction, which is larger: the energy released when making bonds or the energy used to break them?
the energy released by forming bonds > the energy used to break them
in an endothermic reaction, which is larger: the energy released when making bonds or the energy used to break them?
the energy released by forming bonds < the energy used to break them
what is a chemical bond’s bond energy?
energy associated with the chemical bonds, which slightly varies depending on the compound
(may appear in calculations for energy changes)
how is the overall energy change of a reaction calculated?
overall energy change = (sum of the energy needed to break bonds in the reactants) - (sum of energy released when bonds are formed in the products)
what do electrical cells contain?
chemicals which react to produce electricity
what is an electrochemical cell?
two different metal electrodes (as they should be able to conduct electricity)
in contact with an electrolyte (to set up a charge difference between the electrodes)

what does the potential difference of a cell depend on?
type of electrodes
difference in reactivity between the electrodes - bigger the difference, the bigger the V
the electrolyte used
how is a battery formed?
by connecting 2 or more cells together in series
why can rechargeable cells/batteries be recharged?
their reactions are reversible - can be reversed by connecting them to an external electric current
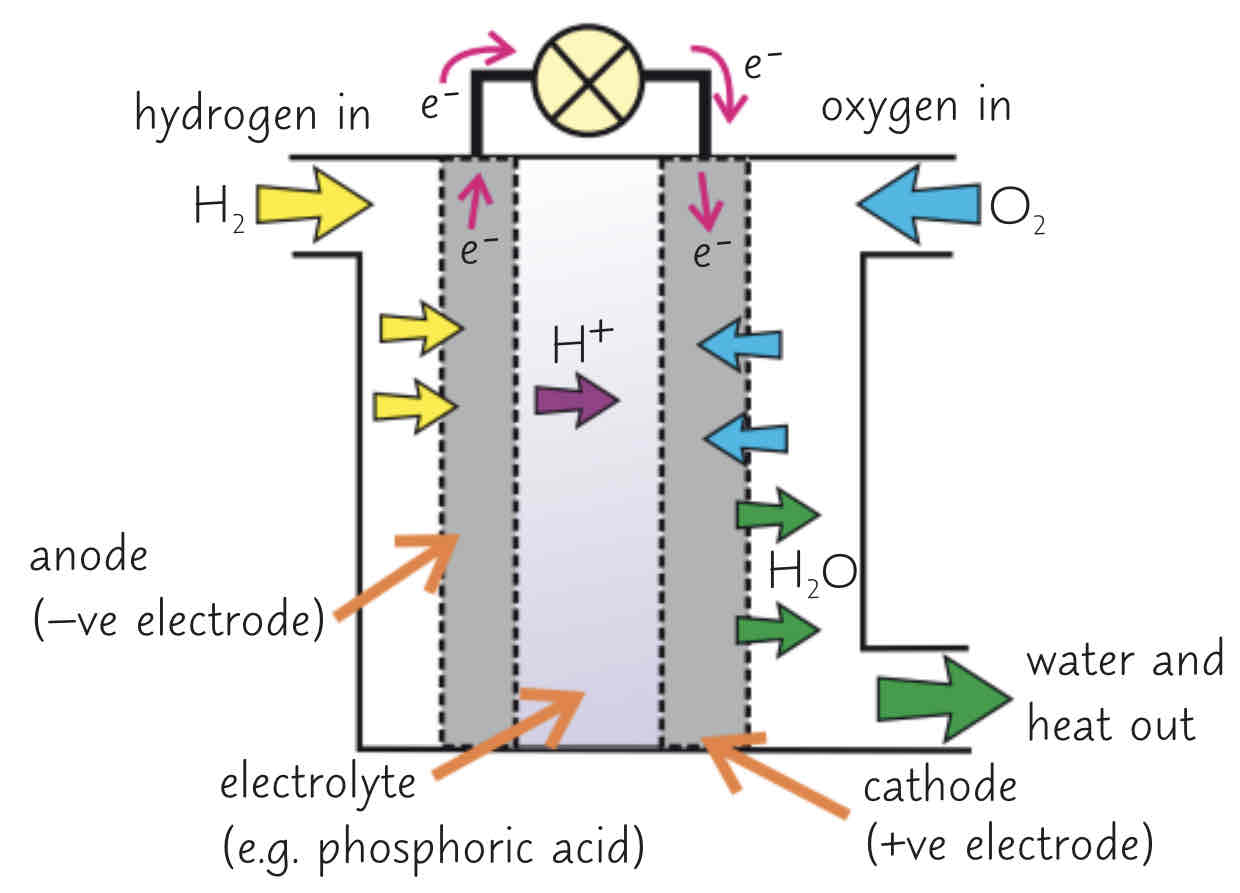
what is a fuel cell?
electrical cell supplied with an external fuel source (e.g. hydrogen) and oxygen/air
the fuel is oxidised electrochemically within the fuel cell to produce a potential difference
(pictured: a hydrogen fuel cell)
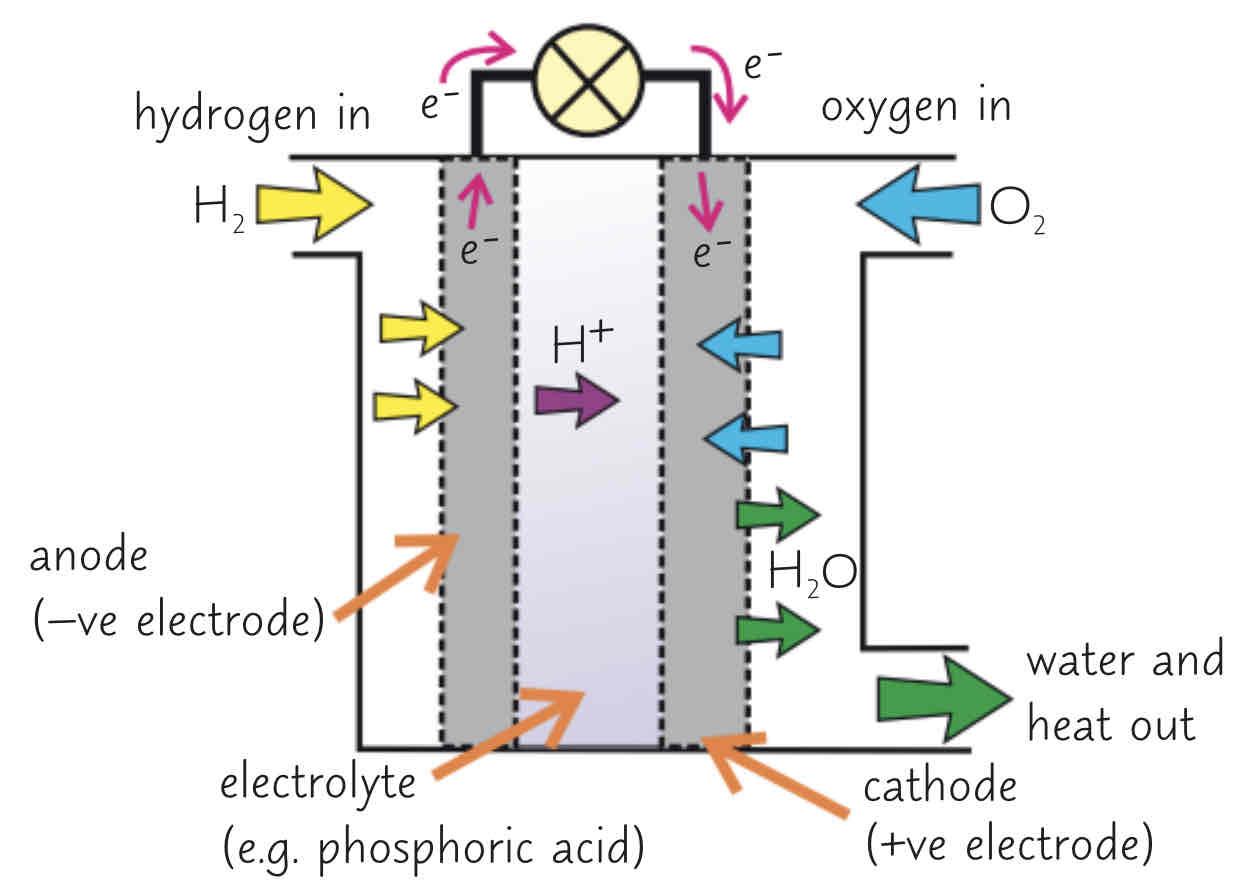
what is the overall reaction in a hydrogen fuel cell?
the oxidation of hydrogen to produce water (2H2 + O2 → 2H2O)
what are the half equations for the reactions at the positive and negative electrode of the fuel cell?
positive: H2 → 2H+ + 2e- (oxidation)
negative: O2 + 4H+ + 4e- → 2H2O (reduction)
evaluate the use of hydrogen fuel cells as opposed to rechargeable batteries
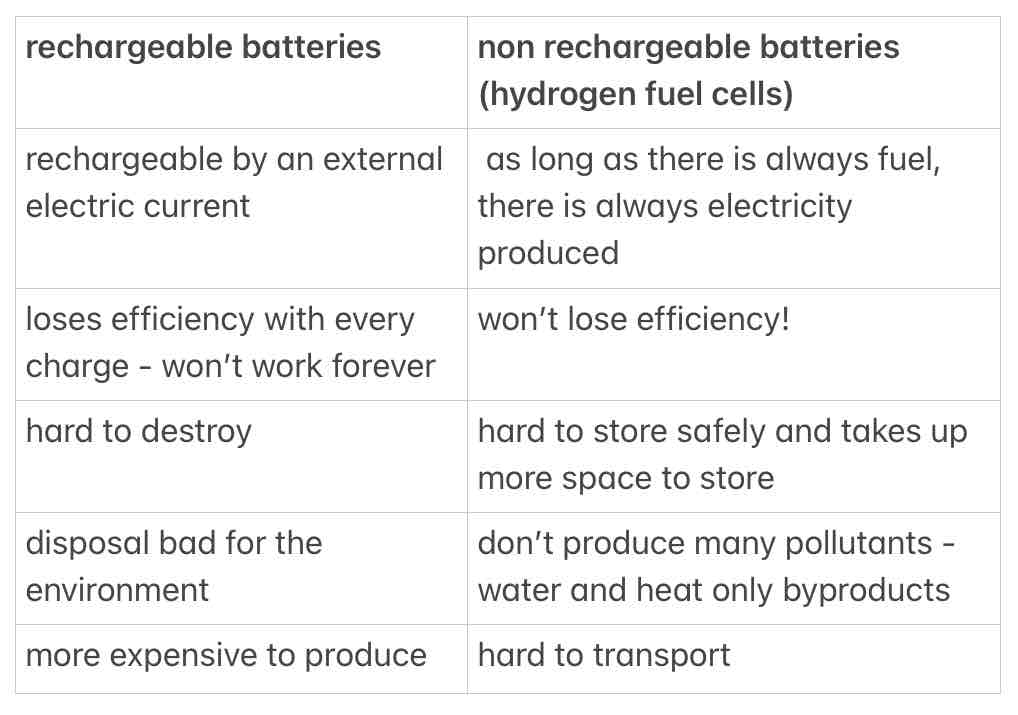
when do chemical reactions stop in a non rechargeable cell?
when one of the reactants has been used up
give 2 examples of non rechargeable cells/batteries
alkaline batteries
fuel cells
explain the process of producing electricity from a fuel cell (words)
electrolyte often an acid, e.g. phosphoric acid
electrodes often porous carbon w/ catalyst
hydrogen goes into the negative electrode compartment and oxygen goes into the positive electrode compartment
at the negative electrode, hydrogen is oxidised - it loses electrons to produce H+ ions (H2 + 2H+ + 2e-)
H+ ions in the electrolyte move to positive electrode
at the positive electrode, oxygen is reduced - it gains electrons from the cathode and reacts w/ H+ ions (from the acidic electrolyte) to form water (O2 + 4H+ + 4e- → 2H2O)
electrons flow through an external current from negative to positive electrode (this is the electric current)
the overall reaction is hydrogen + oxygen → water (2H2 + O2 → 2H2O)
it is a redox reaction as there is both oxidation and reduction