NUTR*4090 Functional Foods and Nutraceuticals - Midterm 2
1/33
Earn XP
Description and Tags
Lecture 8-?
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
What are the 3 types of human studies?
Observational
Intervention
Meta-analysis
What is the purpose of observational studies?
measure associations between foods or food components and disease —> however it cannot determine cause & effect
reflect a “free-living” situation
generate hypotheses and associations to test in interventional studies
What are the 3 types of observational studies?
Cohort studies (prospective)
Case-control studies (retrospective)
Cross-sectional studies (prevalence)
Properties of cohort studies
most reliable
longitudinal - exposure to FFN —> development of disease
incidence of disease in subjects who consumed a food vs those who do not consume the food
Properties of case-control studies
retrospective - development of disease —> exposure to FFN
Key assumption: disease does not affect eating habits
subjects w/ disease are compared to subjects w/o disease based on past dietary habits
Properties of cross-sectional studies
collect info on food consumption at a single point in time in individuals w/ and w/o a specific disease
“snapshot” in time
What are the pros and cons of human observational studies?
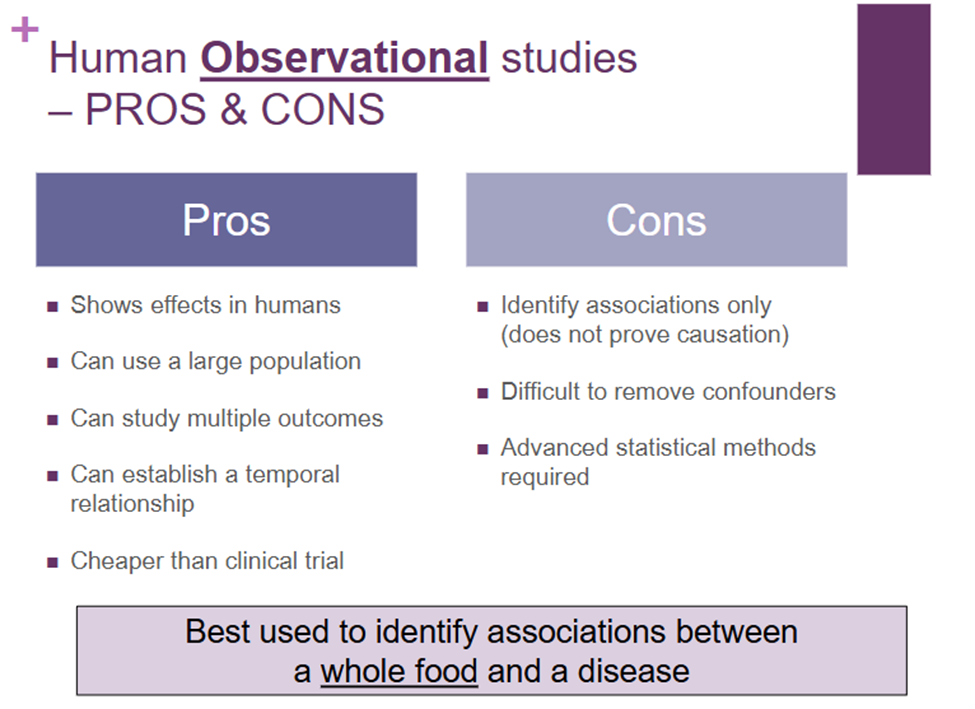
How are intervention studies done?
subjects are provided w/ a food or food component of interest to consume
researchers measure development of disease or biomarkers
typically —> experimental group vs control group
Evaluating human intervention studies

How should we deal with conflicting evidence?
subject characteristics (e.g. age, gender, ethnicity, body weight, etc.)
study design issues (e.g. sample size, duration, randomization, etc.)
What are the pros and cons of human intervention studies?

What are the specific effects of therapeutics?
talks about what happens to the body
influenced by factors such as dose, duration, pharmacodynamics, etc.
What are the non-specific effects of therapeutics?
influenced by factors such as natural history of the diseases, expectations and belief in the treatment, etc.
e.g. placebo/nocebo effects
Overall response results from the combination on specific effects and non-specific effects (T/F)
True
What is the placebo effect?
beneficial
occurs when persons w/ a specific illness demonstrate clinical improvement when treated w/ an inert substance
everything but the bioactive substance should be identical (e.g. taste, texture, visual, etc.)
Mechansism behind placebo effect
repeated exposure to neurosensory suggestions
reinforced by verbal suggestions
Pavlovian conditioning
What is the nocebo effect?
harmful
detrimental outcome associated w/ intervention (opposite of placebo)
harmless substance that when taken by a patient is associated w/ harmful effects due to negative expectations or the psychological condition of the patient
Mechanisms behind nocebo effect
may be caused by factors such as distrust of the medical personnel or lack of confidence in a treatment
What are some possible contributing factors to placebo and nocebo effect?
time spent w/ practitioner
claims made by practitioner
money spent for treatment
environment
physical manipulations
personalized approach
belief that treatment is free of adverse effects
disillusionment w/ conventional treatment
Is selling stuff that cause a placebo effect acceptable?
context dependent —> can be helpful but also in part deceiving the consumer
data supporting the products is minimal
What does CONSORT stand for?
consolidated standards of reporting trials
What are the CONSORT guidelines?
its initiatives were developed to improve reporting of randomized trials
its statement provides minimum set of recommendations for reporting RCTs
It is more difficult to develop a placebo for an herbal product (T/F)
True
lack of blinding may influence response rate
How to select an herbal placebo product?
same or closely resembles the product found to be effective in clinical trials (e.g. same brand, plant species, preparation, etc.)
must be approved by Health Canada (should have NPN)
found on Licensed Natural Health Product Database
scoring system
- rating solely on scientific evidence
- based on safety, effectiveness and product qualityCochrane Review
- systematic reviews of primary research in human health care and health policy
- internationally recognized as the highest standard in evidence-based health care
- investigate the effects of interventions for prevention, treatment and rehabilitation
What is homeopathy?
Alternative medical system
developed by Samuel Hahnemann in Germany
Alternative to harsh therapies
e.g. chinchona bark
Homeopathic substances are approved by Health Canada (T/F)
True
FDA and HC regulate homeopathic remedies (T/F)
True
includes safety and efficacy
What are the 2 principles of homeopathy?
“like cures like”
—> disease can be cured by the substance that produces similar (disease) symptoms in healthy peoplelaw of minimum dose
—> lower dose = greater effectiveness (more diluted)
—> “dose makes the poison”
Where do homeopathic remedies come from?
derived from plants, animals and minerals
final diluted preparation = nosodes
extract that is diluted to create the nosode = tincture
What is the term for the final diluted preparation?
Nosodes
What is the term for the extract that is diluted to create the nosode?
Tincture
Homeopathic treatments/remedies are highly individualized (T/F)
True
remedies based not only on symptoms but lifestyle, emotional and mental states
What is the concept of potentisation?
more diluted = more effective (law of minimal doses)
Why are homeopathic substances regulated by Health Canada?
as long as they are free of negative components on the monographs
e.g. bacteria and heavy metals