Material Science, Materials Science
1/290
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
291 Terms
eutectic
-the lowest temperature at which two solids are at equilibrium w/ liquid phase
peritectic
a three phase reaction where a solid phase and liquid phase transform to a single solid phase upon cooling
Austenite
One of the allotropes of iron, also known as gamma iron. It is formed when iron is between 912 ° and 1,394 °C and has a face-centred cubic structure. Also found in carbon steel.
Allotropy
The ability of a substance to exist in more than one phase in the solid (or indeed, liquid and gaseous) state; the possibility of the existence of two or more different crystal structures for a substance (generally an elemental solid)
Annealing
A heat treatment designed to soften a metal or alloy. Microstructurally, annealing is associated with recovery, recrystallization and/or grain growth; Decrease E, TS, Yield, Increase Duct, No change in dimensions
Bainite
A non-equilibrium phase, usually in steel, which is formed by quenching from the austenite phase. The rate of quenching required is slower than that necessary to form martensite but faster than that which produce the equilibrium phase of pearlite.
BCC
Body-centered cubic packing describes a way in which atoms (considered as hard spheres) pack together to fill space. It comprises a cube of 8 atoms, with another atom at the center.
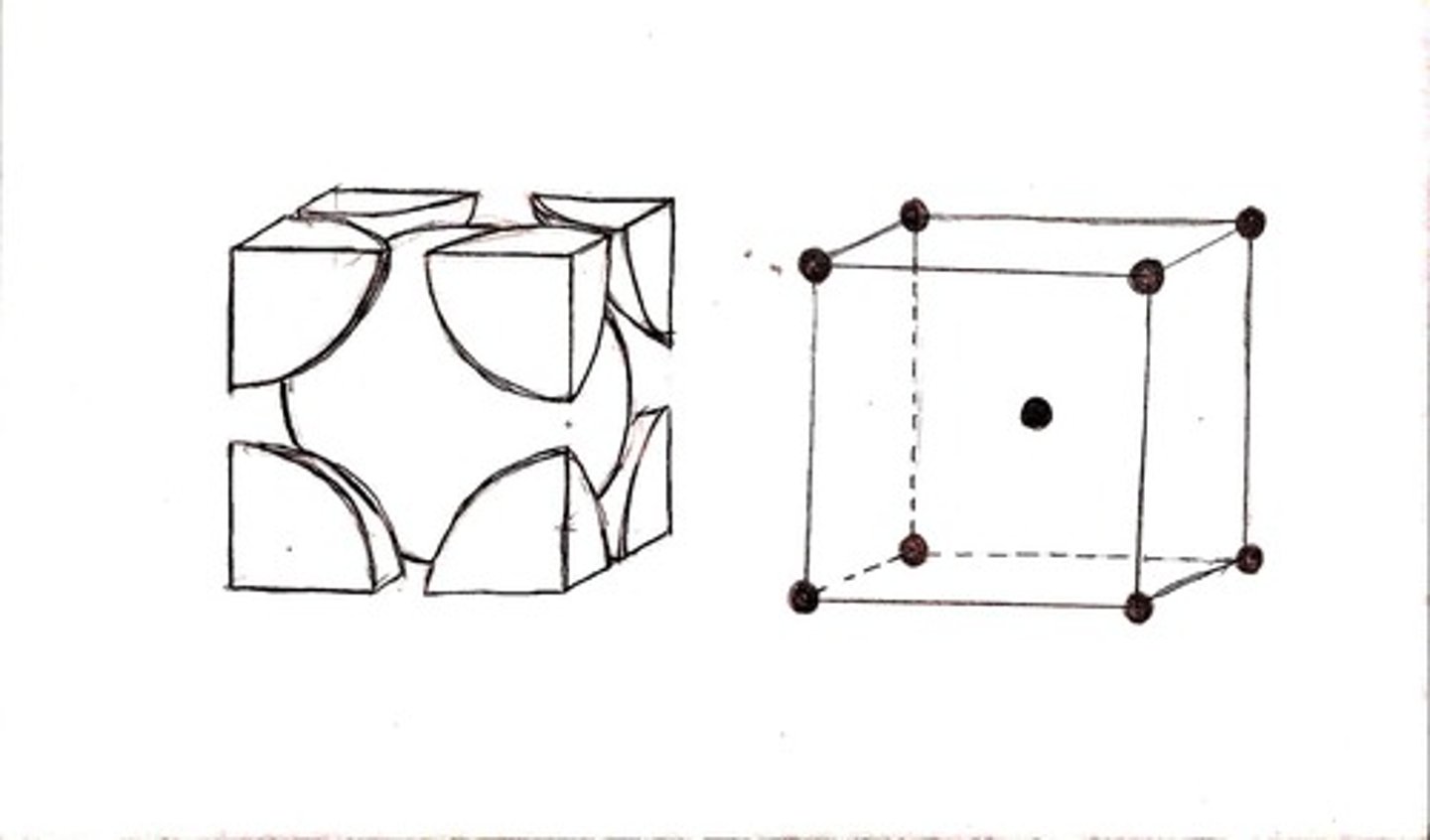
FCC
Face-centered cubic describes a way in which atoms pack together to fill space. The first layer (A) consists of an hexagonal array of atoms. The next layer (B) sits in the hollows of the first layer. The third layer (C) does not duplicate either A or B layer, giving an ABCABC... structure.

Fatigue
The failure of a structure subjected to repeated loading at stress levels below those required to cause general yielding. The process of fatigue may involve the initiation and growth of cracks from stress concentrations. However, the initiation stage is often by-passed since micro-cracks are usually introduced into a structure during processing.
S-N curve
A graph plotting stress amplitude, S versus number of cycles to failure, N for a fatigue test.
Some materials, notably low carbon steels exhibit a fatigue limit, below which stress, failure never occurs. More commonly, no such limit exists and an endurance limit must be defined as the stress required to cause failure after 108 cycles.
Ferrite
Pure iron up to 912 ºC has a bcc structure and is known as alpha ferrite. Between 1394 ºC and the melting point of iron the bcc structure is now known as delta ferrite. Also found in carbon steel.
Fick's first law of diffusion
States that the rate of diffusion, or flux, J of a species is proportional to the concentration gradient.
amorphous
(adj.) shapeless, without definite form; of no particular type or character; without organization, unity, or cohesion
anion
A negatively charged ion
anisotropy
having a different value when measured in different directions
atomic packing factor (APF)
the volume of atoms in a selected unit cell divided by the volume of the unit cell
Bragg's law
nλ=2dsinΘ
cation
A positively charged ion
coordination number
The number of immediately adjacent (i.e. touching) atoms to a given atom is called the:
crystalline
A solid that is made up of crystals in which particles are arranged in a regular, repeating pattern
crystal structure
The arrangement of the atoms in a material into a regular repeatable lattice.
crystal system
any of the seven groups (cubic, hexagonal, rhombohedral, tetragonal, orthorhombic, monoclinic, and triclinic) of crystals
grain boundary
The interface separating two adjoining grains having different crystallographic orientations.
hexagonal close-packed (HCP)
most common non-cubic bravais lattice, 6 atoms form a hexagon on both the top and bottom and a single atom positioned in the center between the two hexagonal rings
isotropic
Having the same property in all directions.
lattice parameters
the edge lengths and angles of a unit cell
Miller indices
A shorthand notation to describe certain crystallographic directions and planes in a material. Denoted by [ ] brackets. A negative number is represented by a bar over the number.
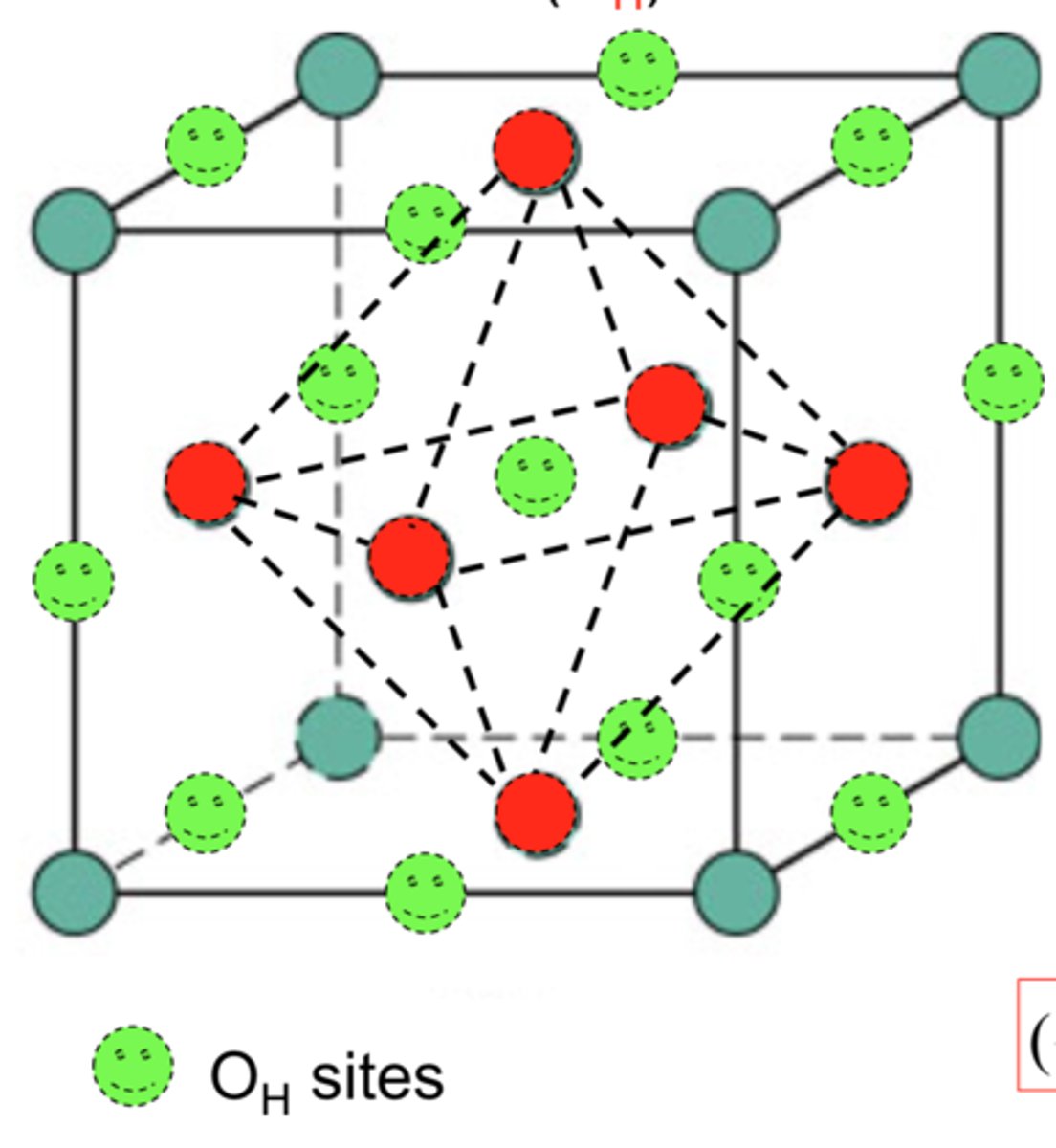
octahedral position
the void space among close-packed, hard-sphere atoms or ions for which there are six nearest neighbors; an octahedron (double pyramid) is circumscribed by lines constructed from centers of adjacent spheres
tetrahedral position
interstitial position where the atoms surrounding the interstitial atom forms a tetrahedron
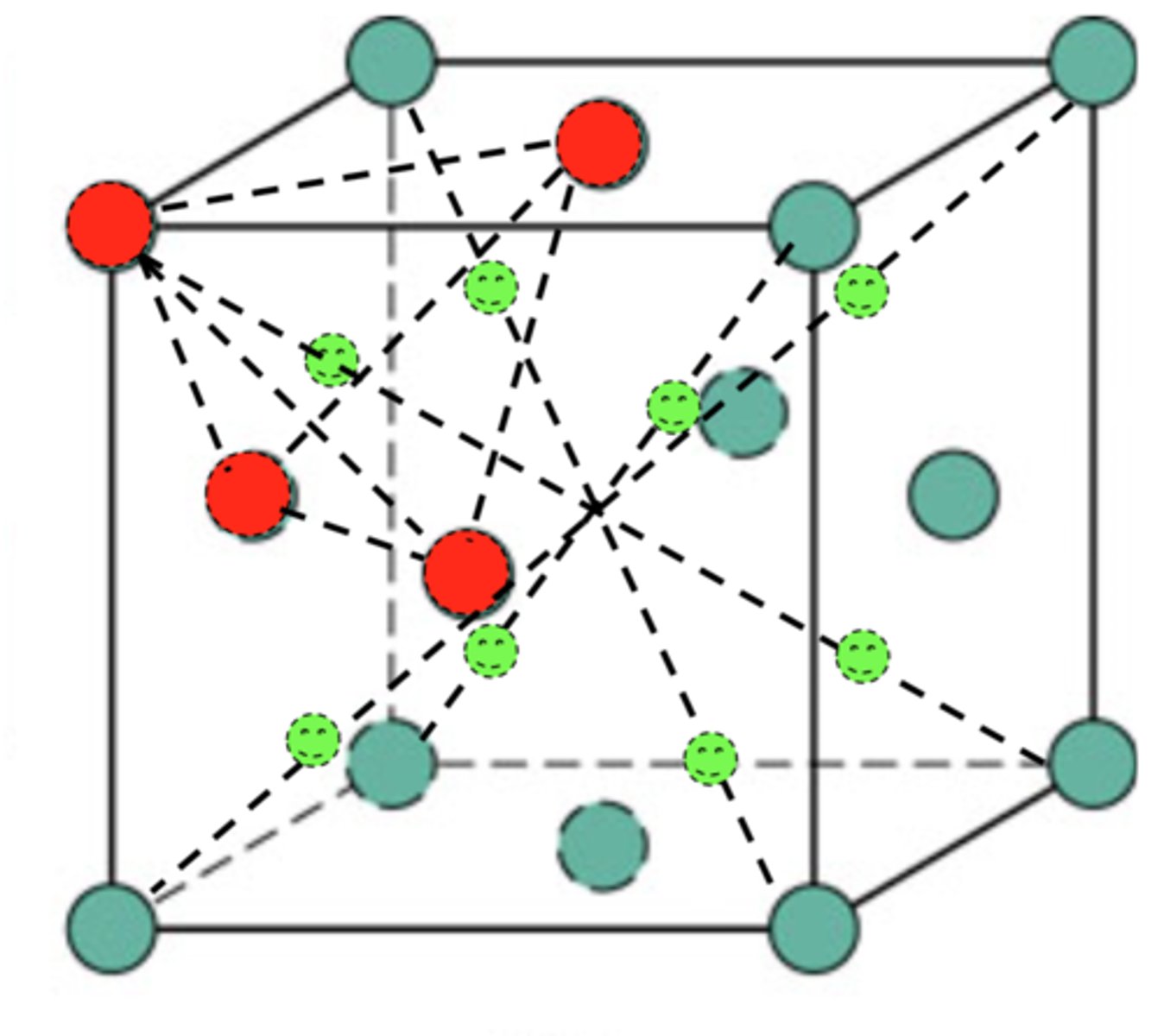
unit cell
A simple arrangement of atoms or molecules which is repeated to develop a solid crystal structure is called unit cell.
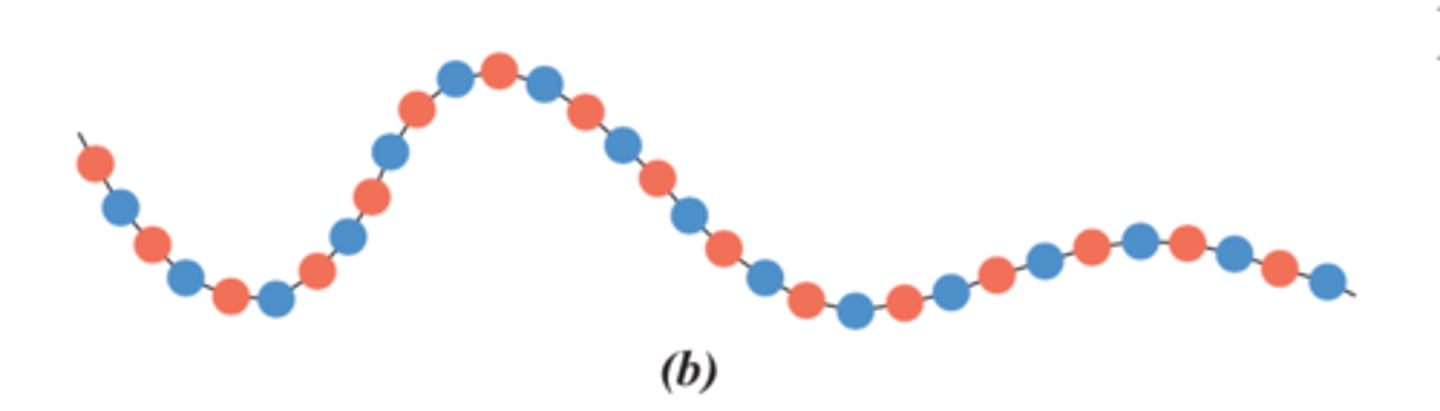
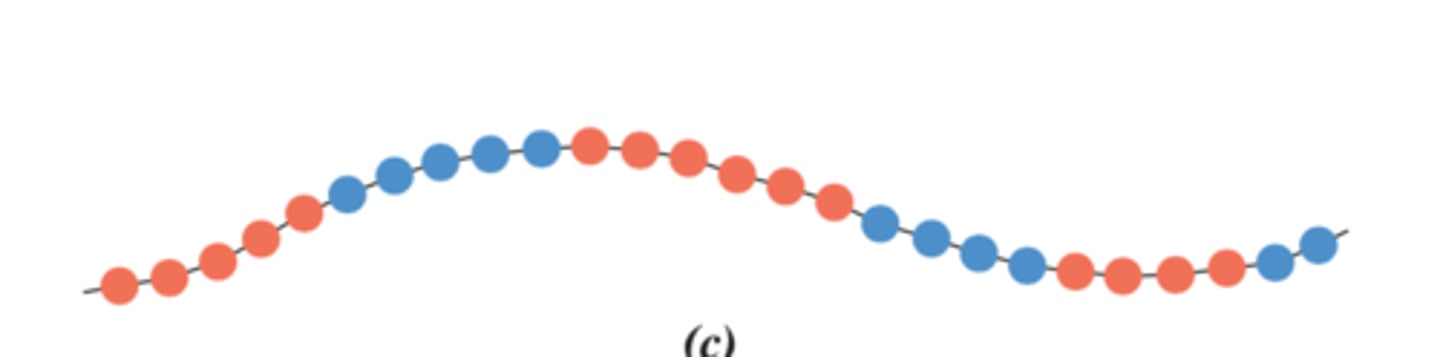
alternating copolymer
Alternating sequence of monomers
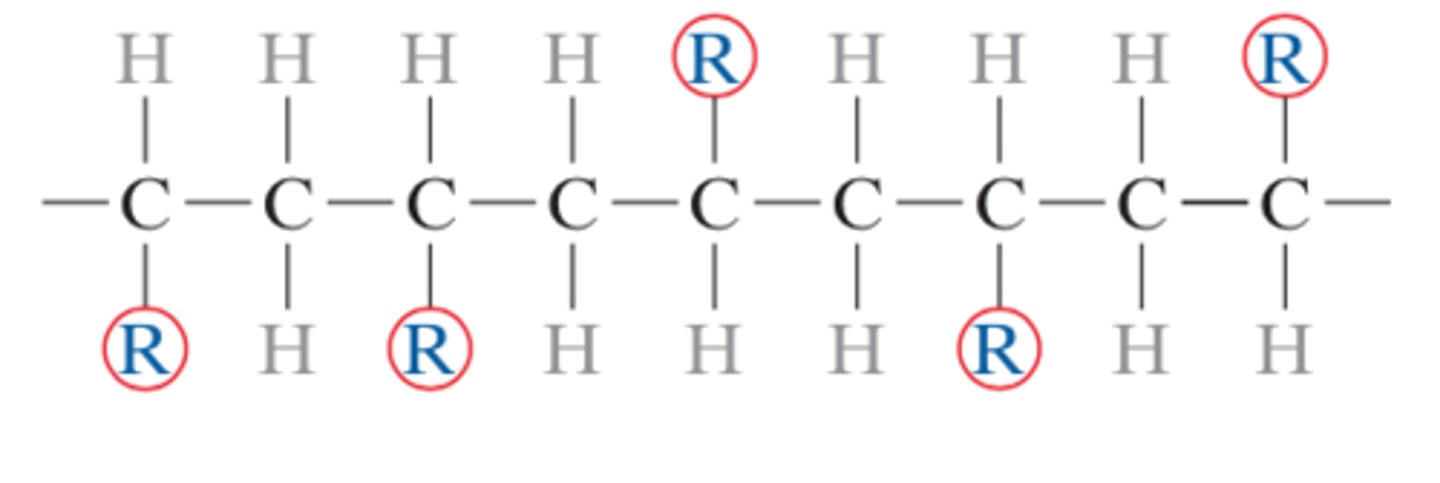
atactic configuration
a type of polymer chain configuration (stereoisomer) wherein side groups are randomly positioned on one side of the chain or the other
block copolymer
identical repeat units are clustered in blocks along the chain
branched polymer
a polymer having a molecular structure of secondary chains that extend from the primary main chains
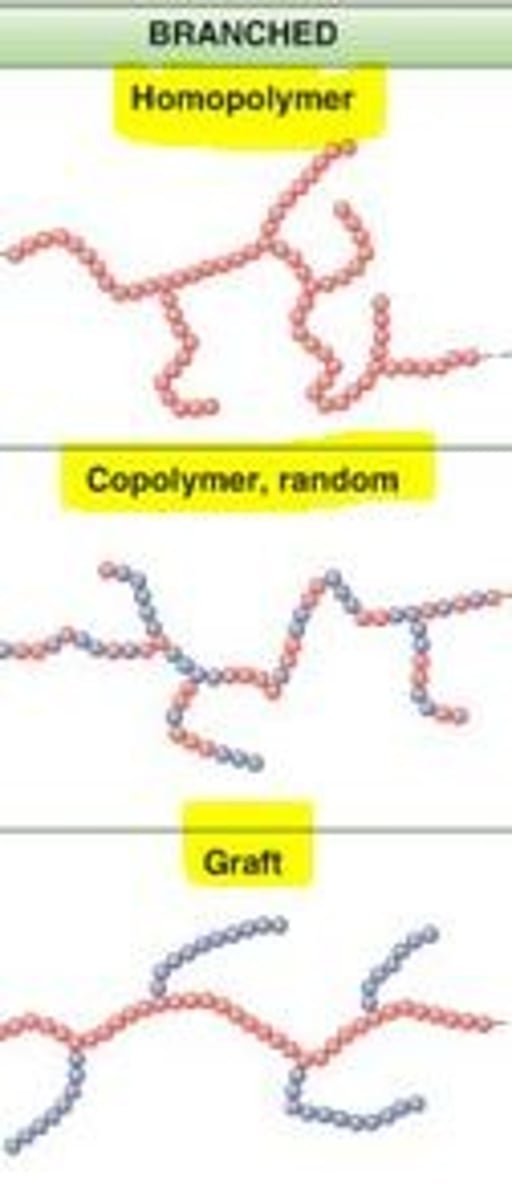
copolymer
a polymer consisting of two or more different monomers
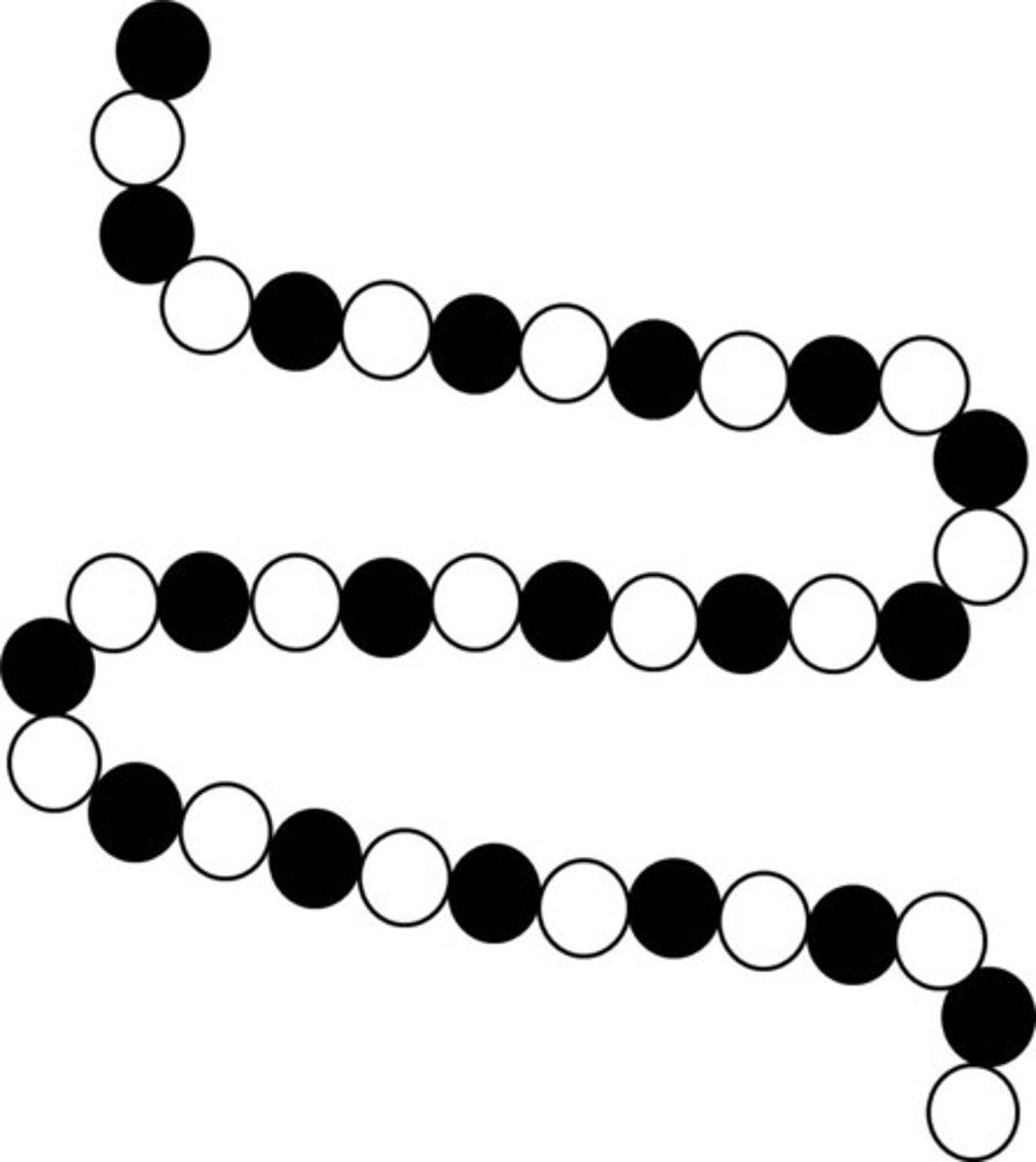
crosslinked polymer
Permanently bonded, tangled polymers. A crosslinked polymer can never melt. Most flexible and is the most dense polymer
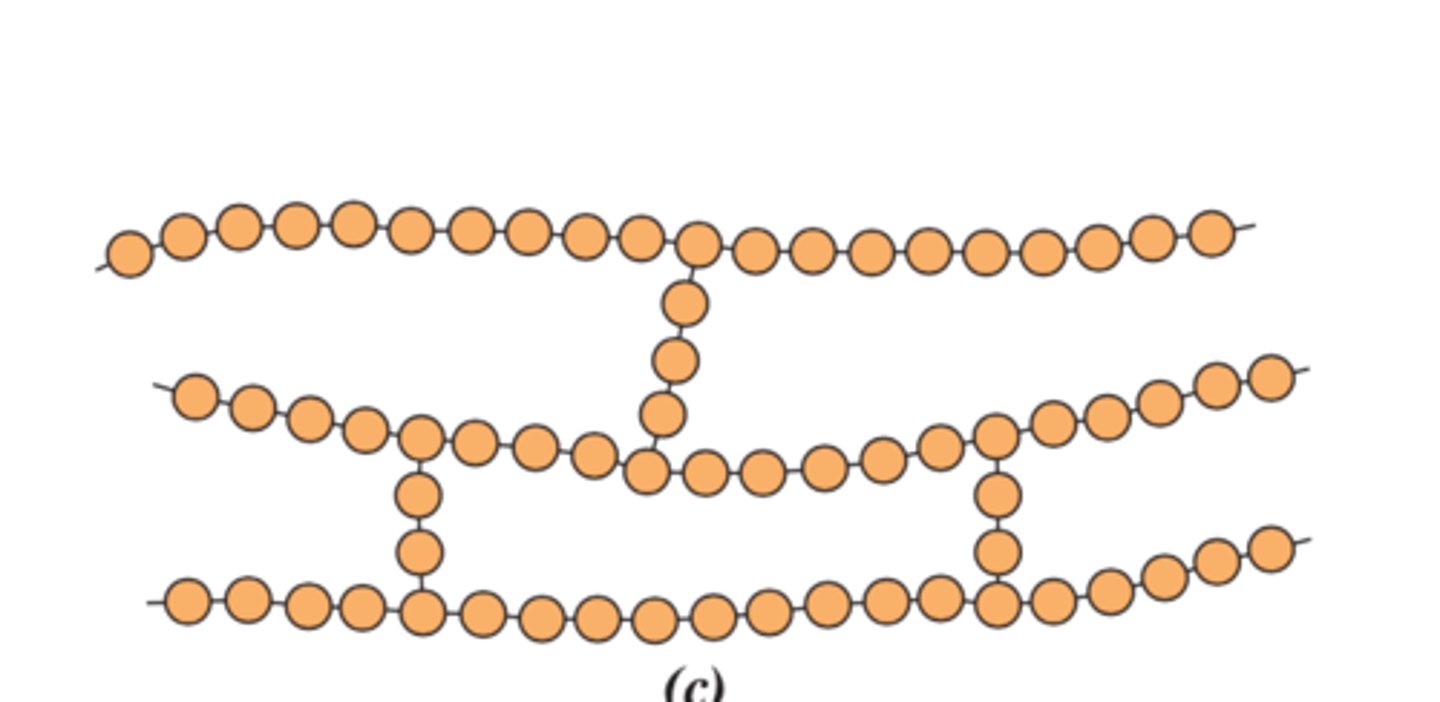
degree of polymerization
number of repeating units within the chain
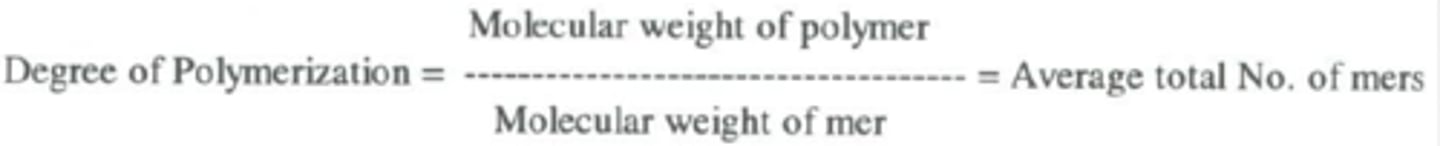
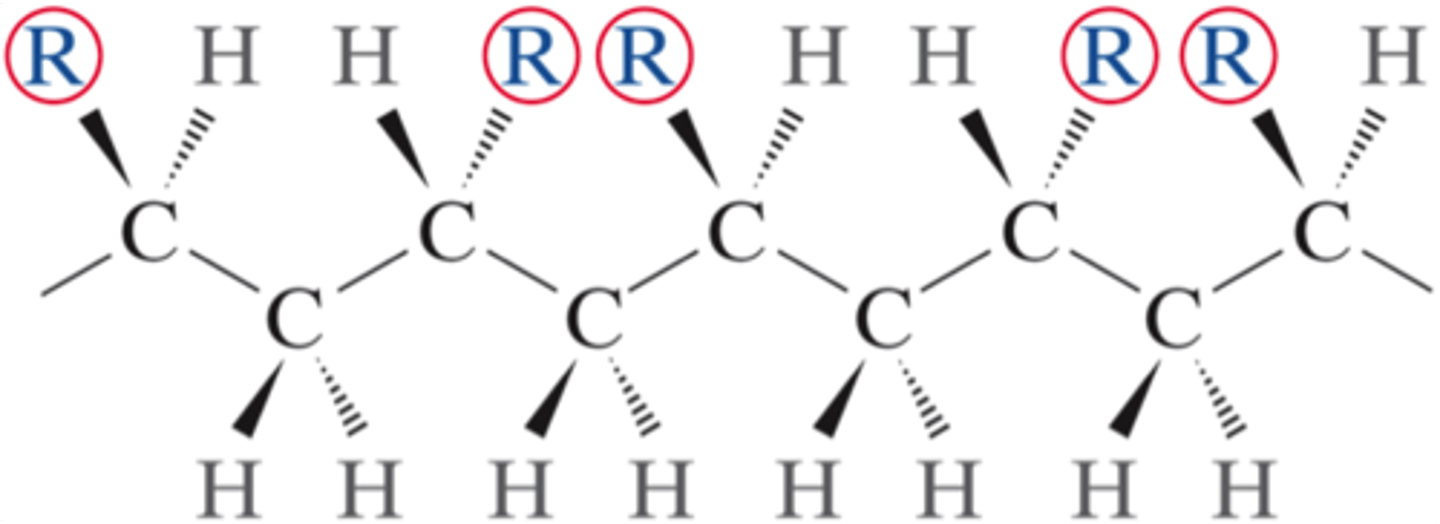
isotactic configuration
a type of polymer chain configuration (stereoisomer) wherein all side groups are positioned on the same side of the chain molecule

linear polymer
polymer in which the molecules form long chains without branches or cross-linked structures
molecular weight
The molecular weight of a compound is the sum of the atomic weights of all atoms in the molecule.
monomer
A simple molecule that can combine with other like or unlike molecules to make a polymer
network polymer
A polymer in which monomers are connected in a three-dimensional cross-linked network.
polymer
A compound consisting of repeated linked monomers.
random copolymer
A copolymer in which different monomers are linked in no particular order.
syndiotactic
In polymers, an orientation where the side groups alternate in a regular pattern from side to side along the backbone of the polymer.
thermoplastic polymer
A polymer that can be melted and solidified multiple times. Conversely, heat-set elastomers and thermosets undergo a crosslinking reaction upon heating to form a 3-D covalently bonded network and cannot be remelted. Thermoplastics include PE, PP, PVC, PS, PMMA, PVDC, PET, PC, Nylon, and Kevlar.
A semi crystalline polymeric material that softens when heated and hardens when cooled. While in the softened state, articles may be formed by molding or extrusion
thermosetting polymer
polymers that undergo a chemical reaction to produce a rigid cross-linked network
alloy
A COMBINATION; A MIXTURE OF TWO OR MORE METALS
atom percent
(at%) the number of moles of an element in relation to the total moles of the elements in the alloy
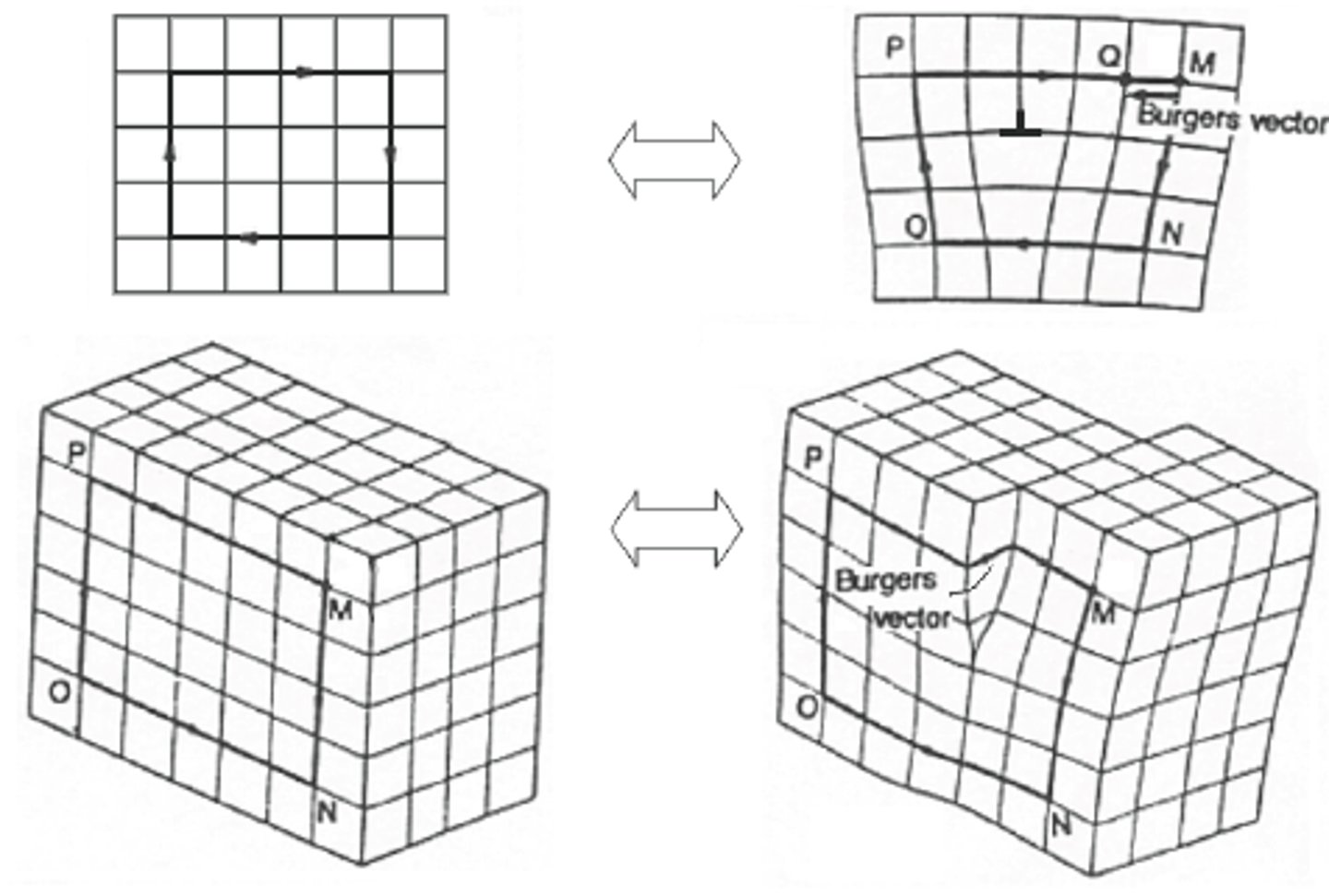
Burgers vector
The direction and magnitude of the slip caused by a single dislocation
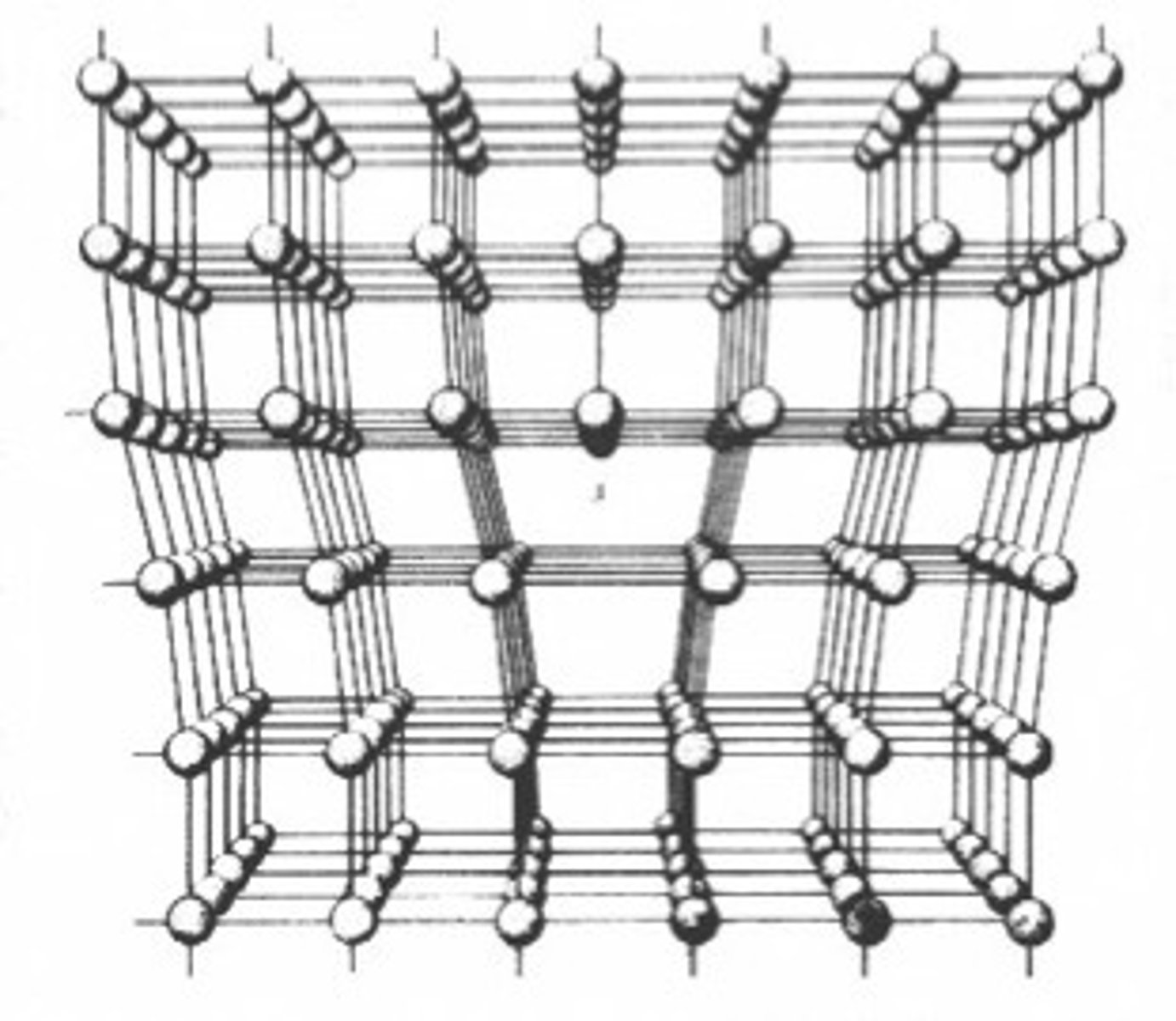
dislocation line
the line extending along the extra partial plane of atoms in an edge dislocation
edge dislocation
a line defect; a slip of the part of crystal over an atomic plane relative to another part is perpedicular to this plane.
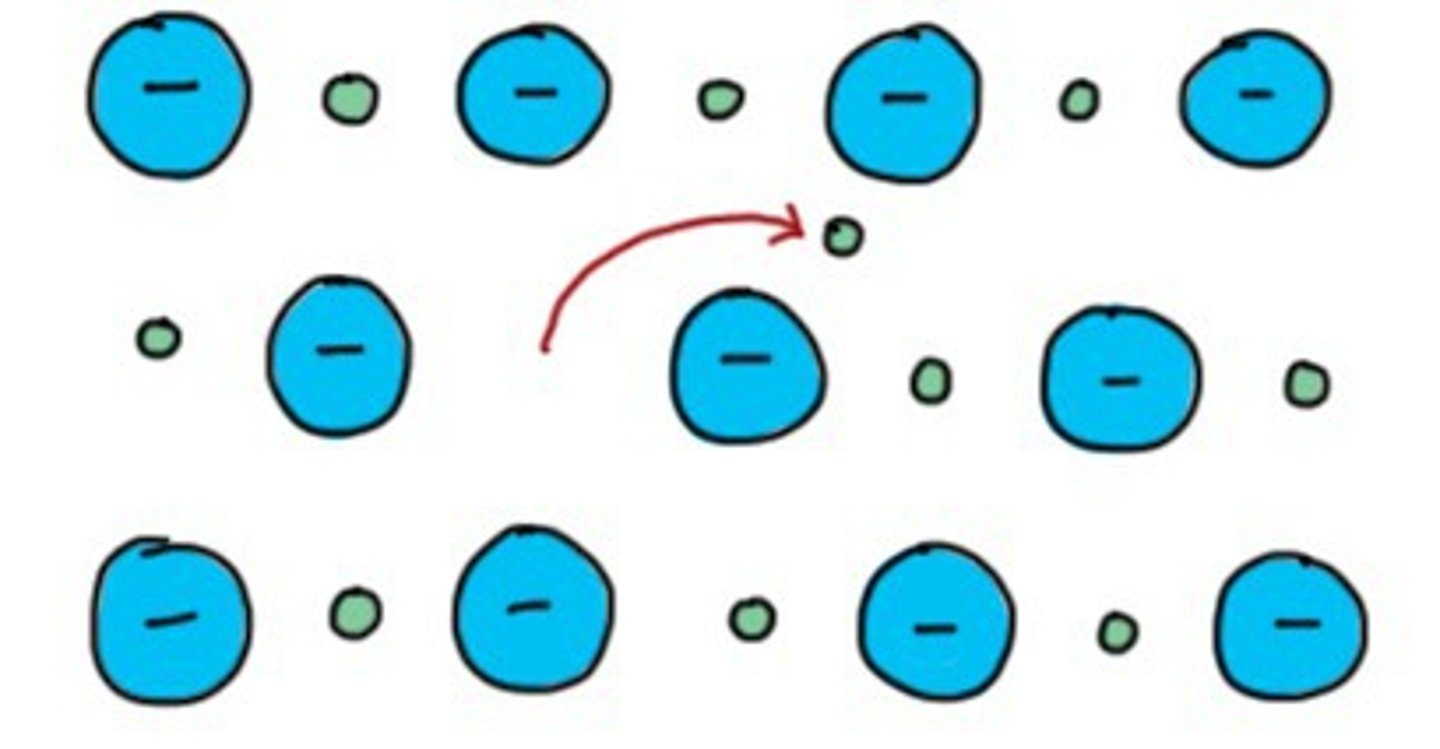
Frenkel defect
point defect found in ceramic materials that occurs when a cation diffuses into an interstitial site on the lattice
interstitial solid solution
a solid solution wherein relatively small solute atoms occupy interstitial positions between the solvent or host atoms.
microstructure
the structural features of an alloy that can be seen under a microscope (ex: grain and phase structure)
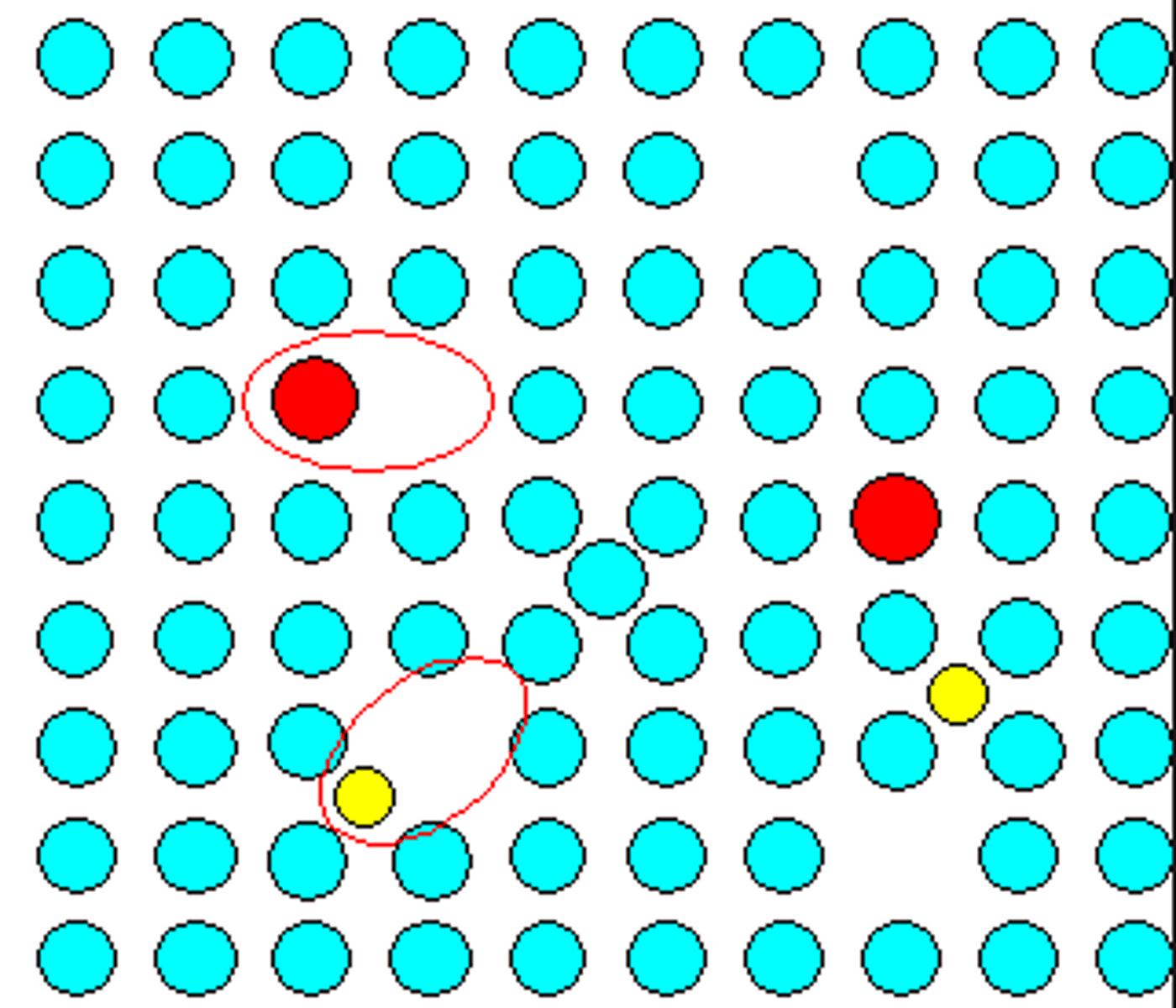
point defect
a flaw in the structure of a material that occurs at a single site in the lattice, such as vacancies, substitutions, and interstitial defects
scanning electron microscope (SEM)
A microscope that is especially useful for the detailed study of the surface of a specimen; electron beams scan the surface of the sample, which is usually coated with a thin film of gold, allowing electrons on the surface to be deleted and translated into an image; result is a 3D topographic image

Schottky defect
small cation and anion vacancy clusters that are formed in ionic solids. The cation to anion ration in these clusters is adjusted to maintain electroneutrality.
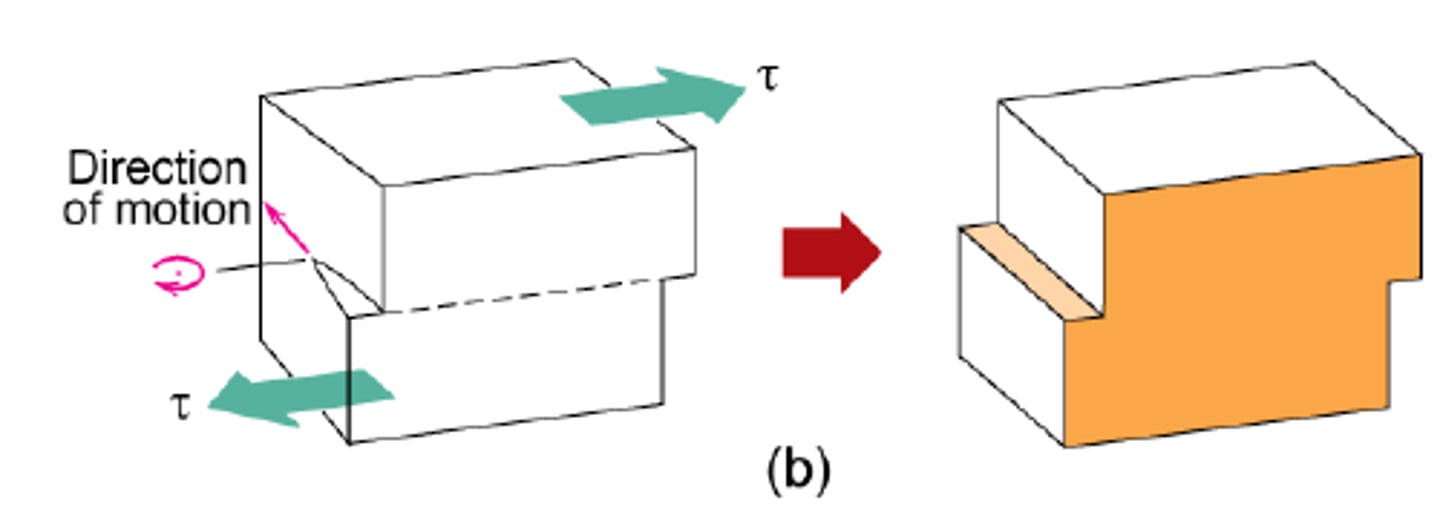
screw dislocation
a line defect in which the Burgers vector is parallel to the dislocation line
self-interstitial
Atom crowded into an interstitial site, that under normal circumstances normally isn't occupied.
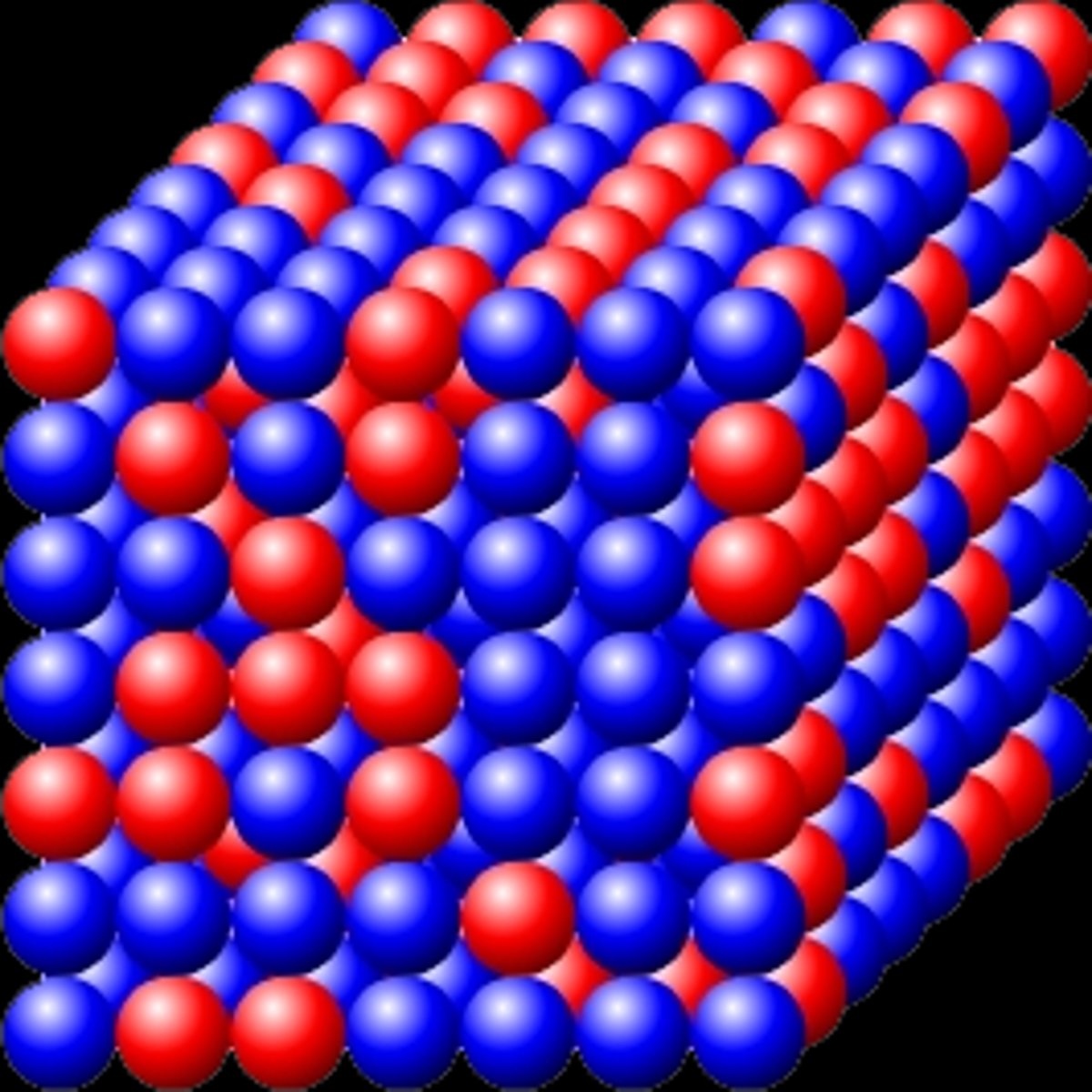
solid solution
Is a metallic substance that is composed of two or more metallic elements.
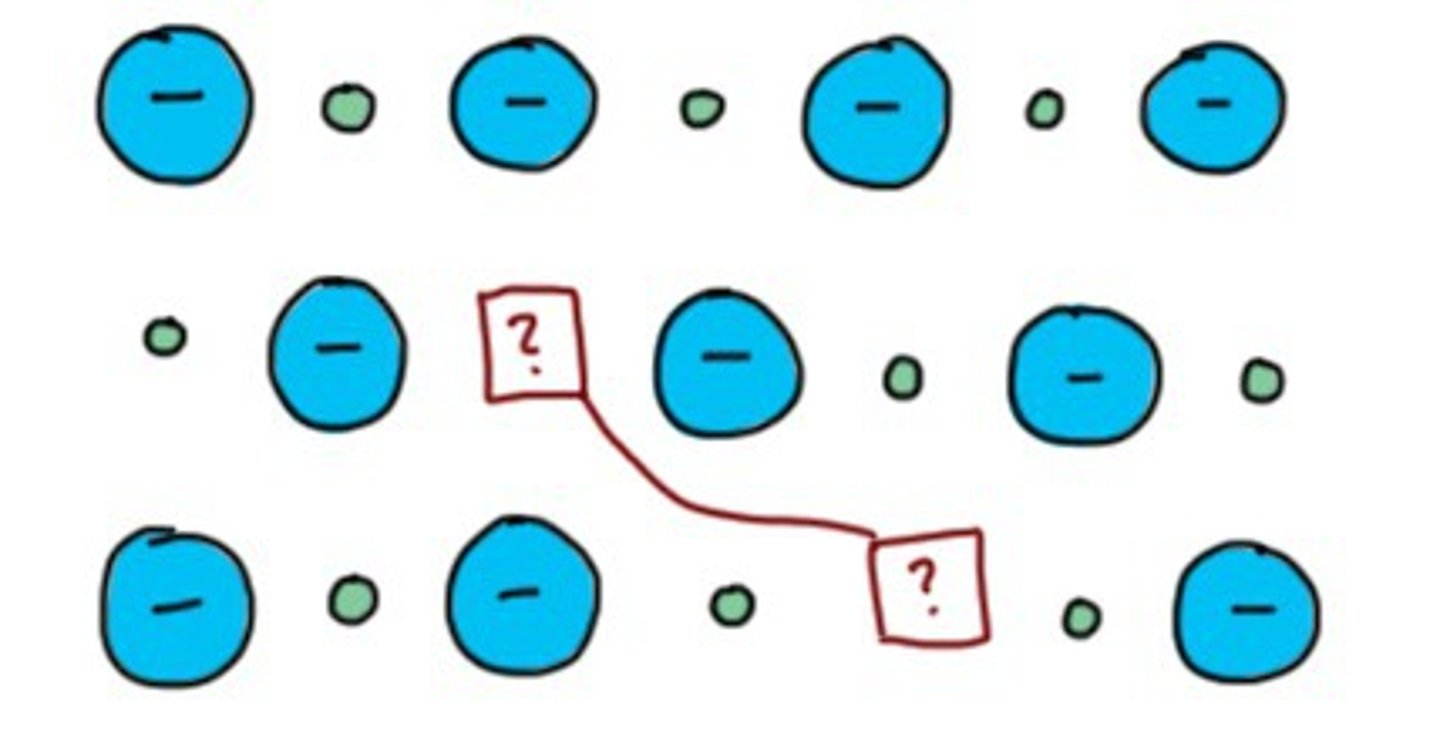
solute
A substance that is dissolved in a solution.
solvent
The dissolving agent of a solution
substitutional solid solution
A solution where a solute atom replaces a host atom on a lattice site in a crystal
vacancy
Type of point defect where there is a vacant lattice site
weight percent
mass of one component divided by the total mass of the mixture, multiplied by 100%
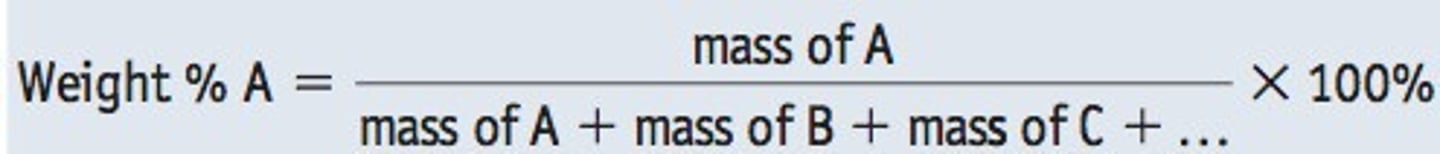
activation energy
Energy needed to get a reaction started
carburizing
produces a high carbon layer on the surface of a metal by exposing the metal to a carbon-containing atmosphere at elevated temperatures, such that carbon atoms diffuse into the surface of the metal.
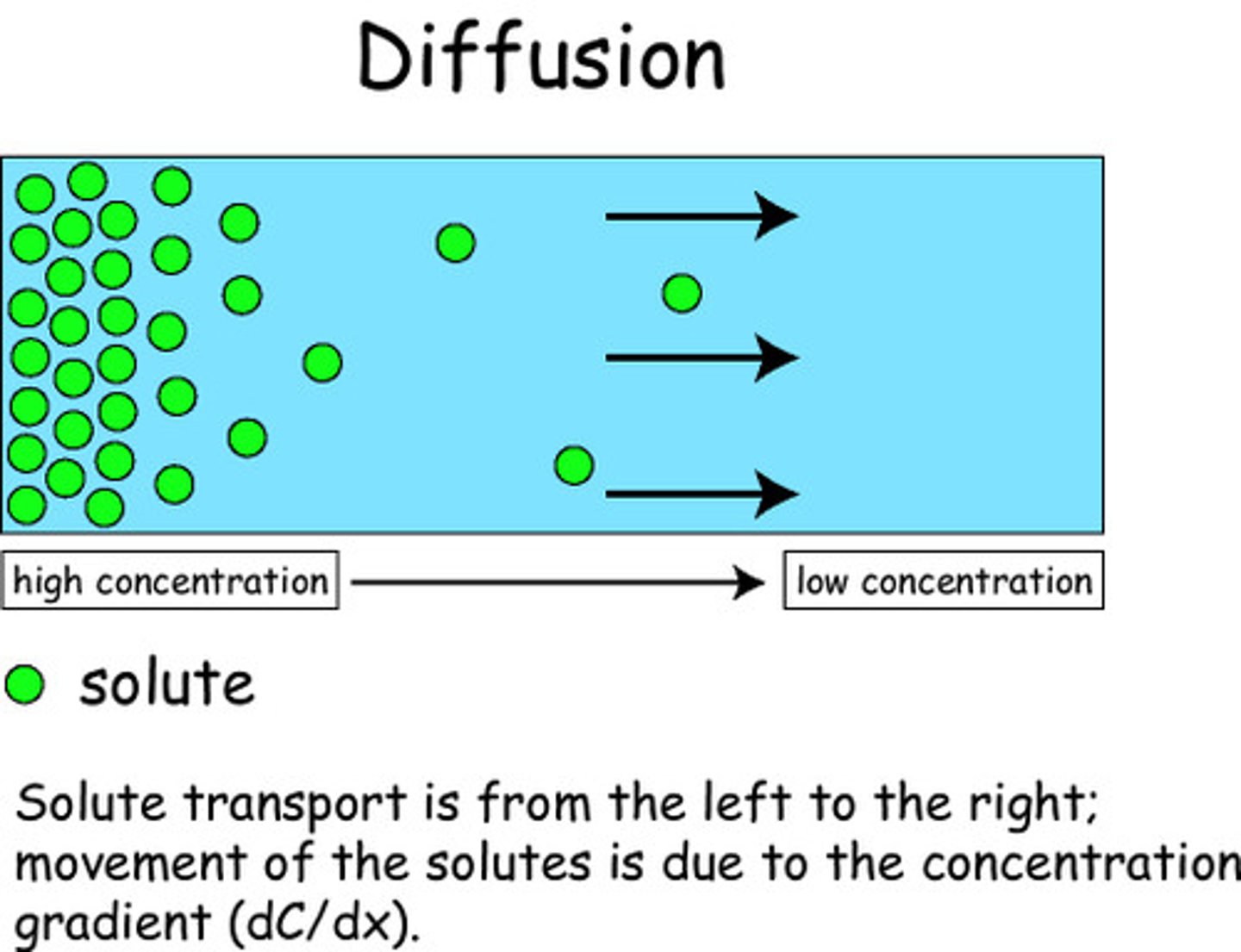
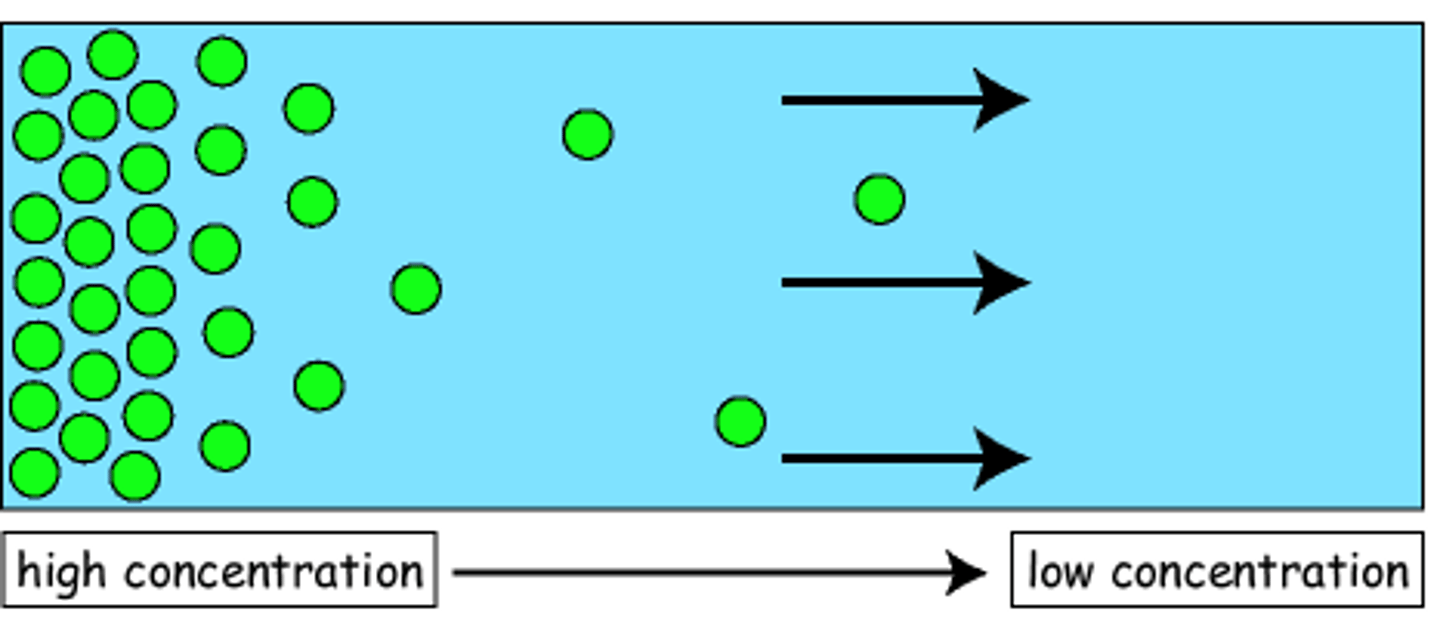
concentration gradient
A difference in the concentration of a substance across a distance
concentration profile
curve of the concentration v. position graph
diffusion
Movement of molecules from an area of higher concentration to an area of lower concentration.
diffusion coefficient
a factor that determines the rate of net movement of a substance by diffusion, it is a property that depends on the particle size of the substance and the nature of the medium in which diffusion is occurring.
diffusion flux
how fast the foreign mass can transfer across and area J=M/At
driving force
A force that tends to encourage change in a particular direction.
Fick's first law
use for steady state diffusion; net flux (#atoms/area-time) of molecules of material A diffusing in the x-direction (dx) because of a concentration gradient (dCa/dx)--> A can diffuse through material B.
Fick's second law
describes the diffusion of atoms when the concentration is changed with time
interstitial diffusion
atoms migrate from an interstitial position to a neighboring one that is empty , this occurs more rapidly than vacancy diffusion because interstitial atoms are smaller and more mobile
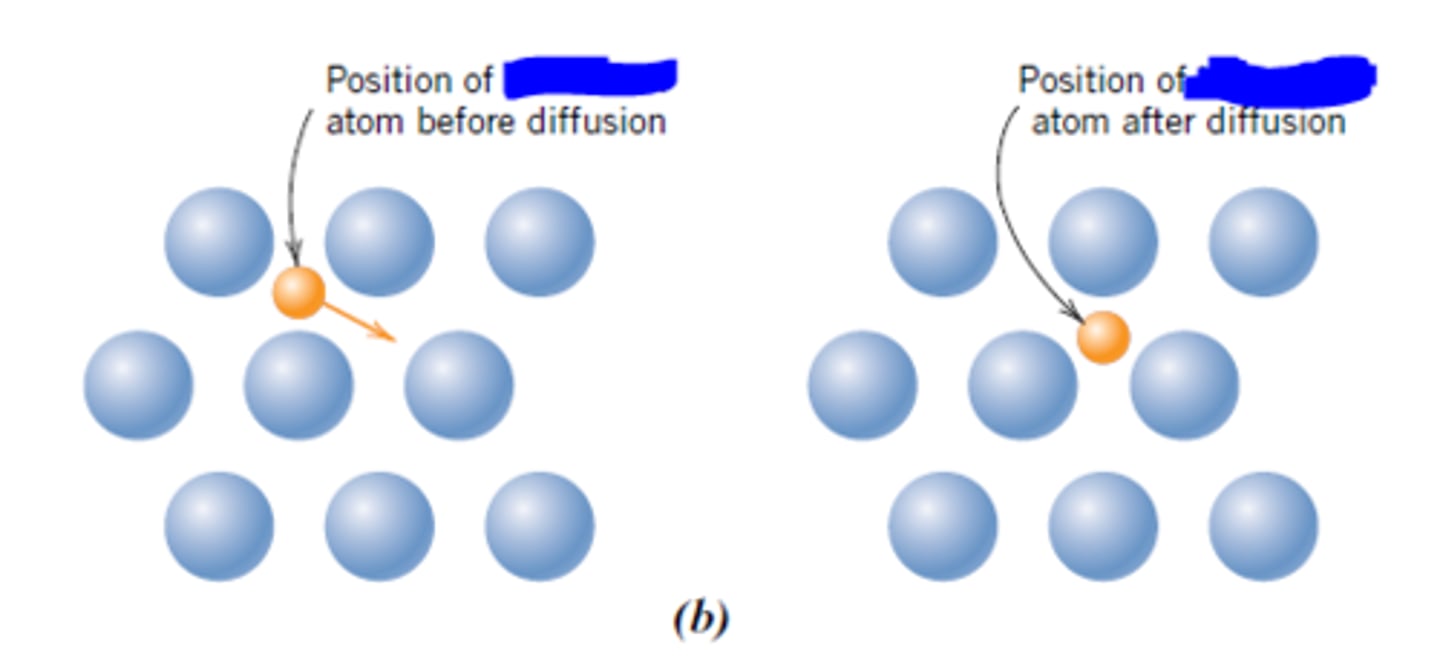
self-diffusion
the mechanism by which a species diffuses in itself
steady-state diffusion
diffusion flux does not change with time
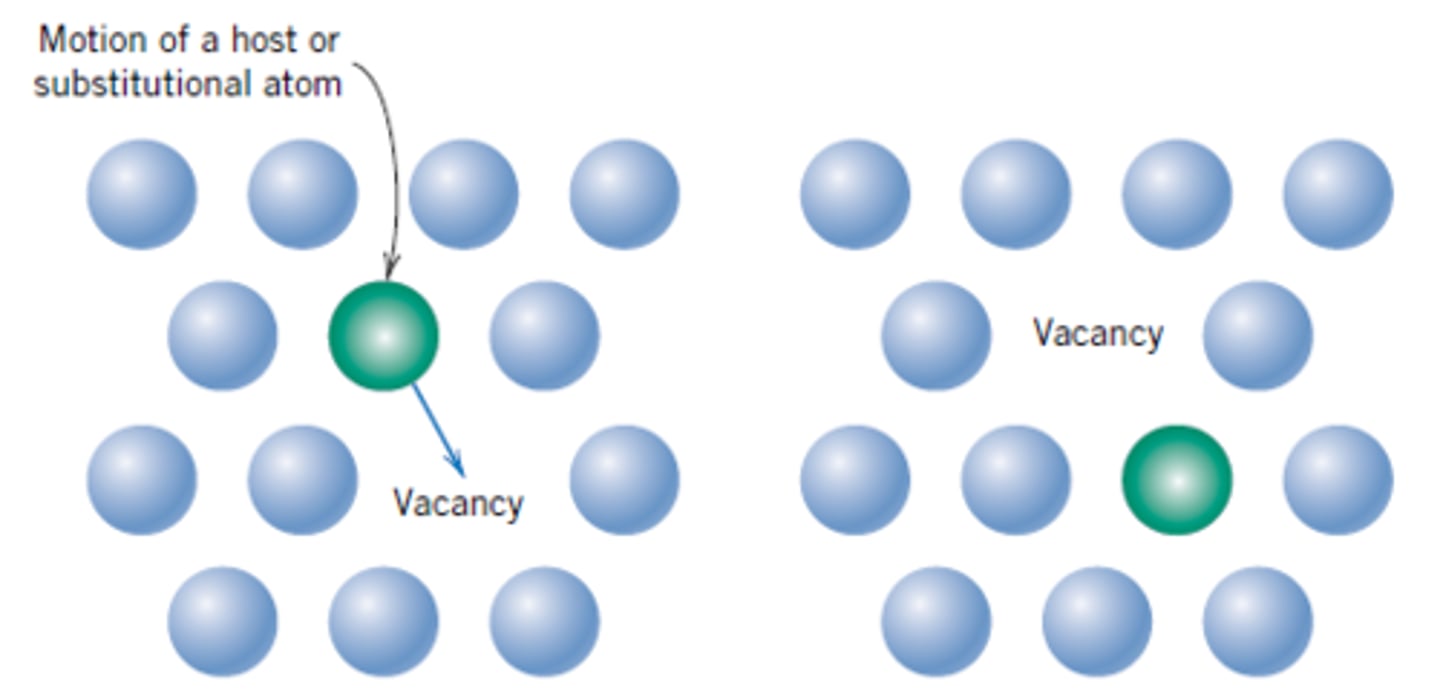
vacancy diffusion
one mechanism involves the interchange of an atom from a normal lattice position to an adjacent vacant lattice site or vacancy
ductility
Ability of a material to stretch or deform under a load without breaking:
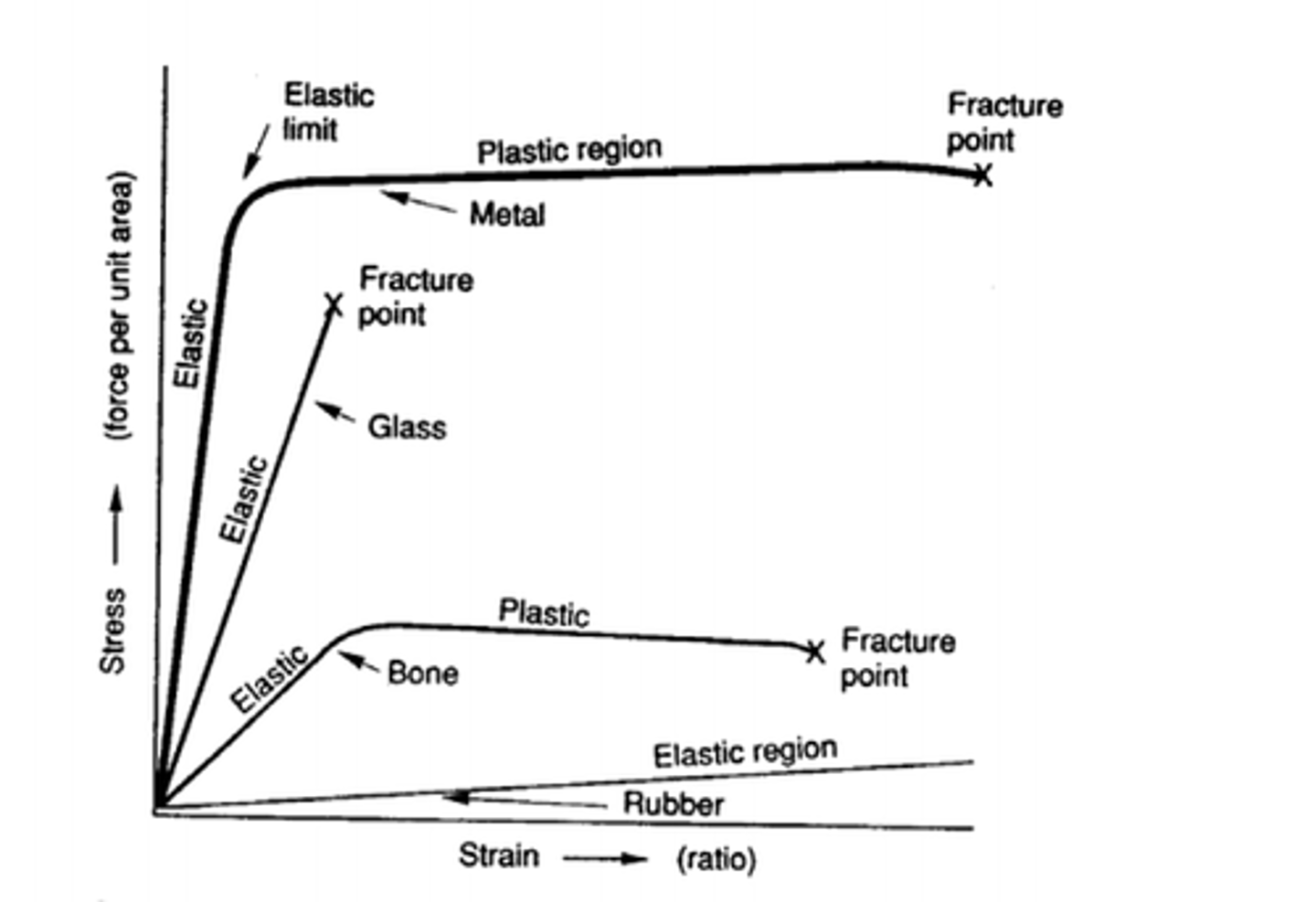
elastic deformation
Occurs when an object changes shape because of a stress is being applied, but snaps back into shape when the stress is removed.
elastomer
a material that can undergo a substantial change in shape via stretching, bending, or compression and return to its original shape upon release of the distorting force
engineering strain
the change in length of sample divided by the original length of the sample
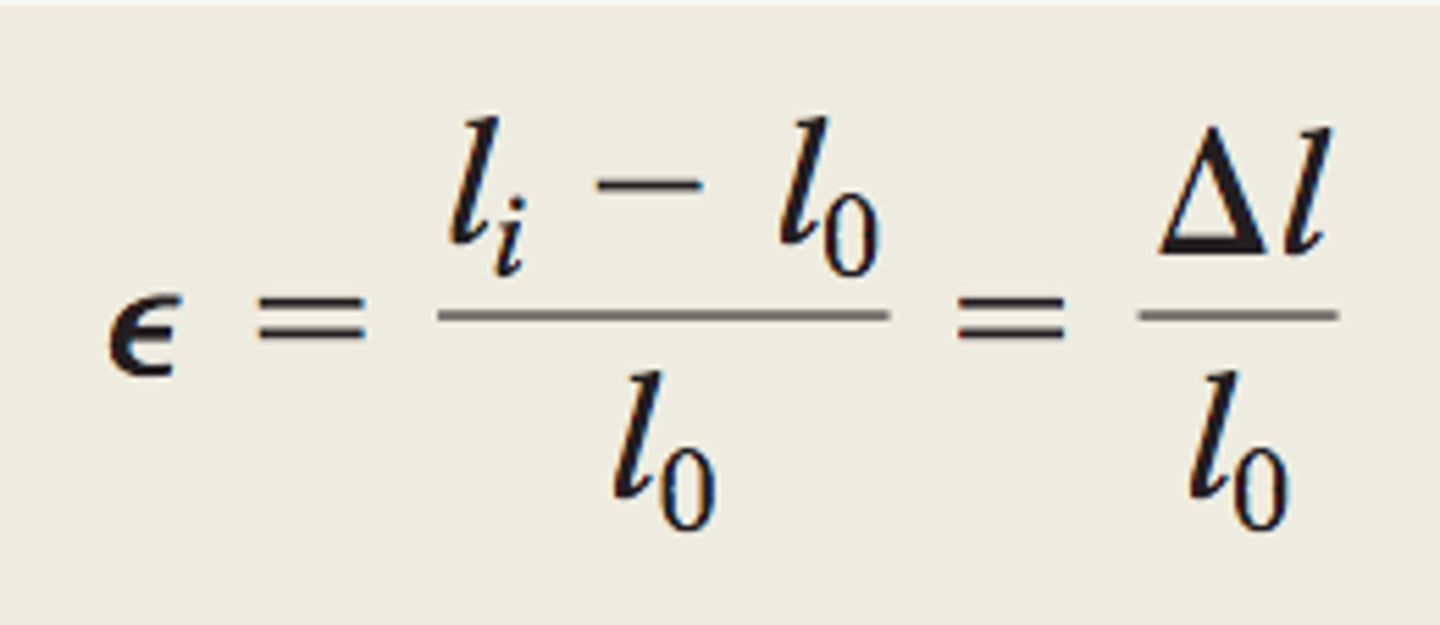
engineering stress
Average force divided by the original cross-sectional area.
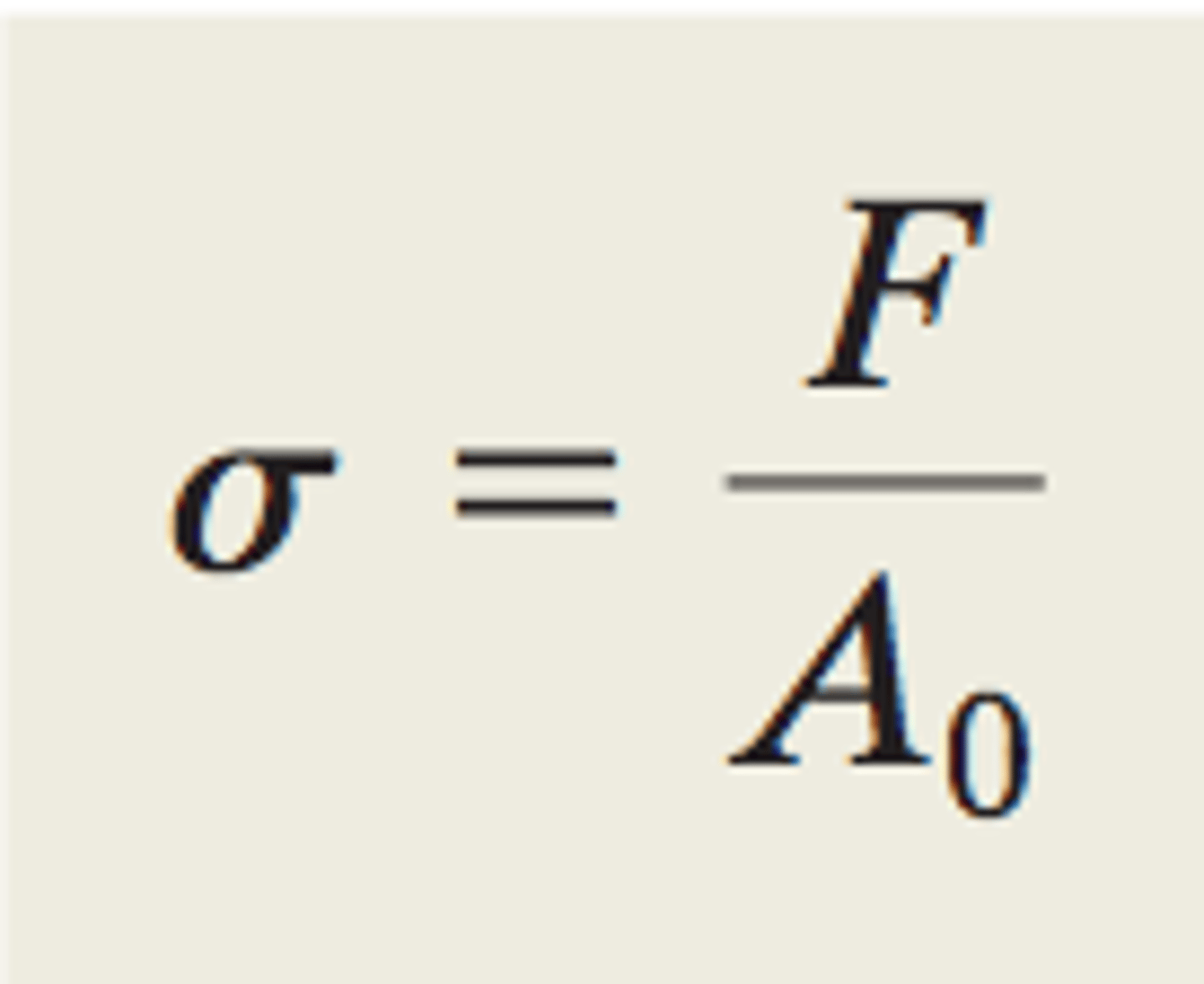
flexural strength
the amount of flexural stress a material can withstand before breaking. measured through the bend test.
hardness
-Refers to various properties of matter in the solid phase that give it a high resistance to its shape changing when force is applied
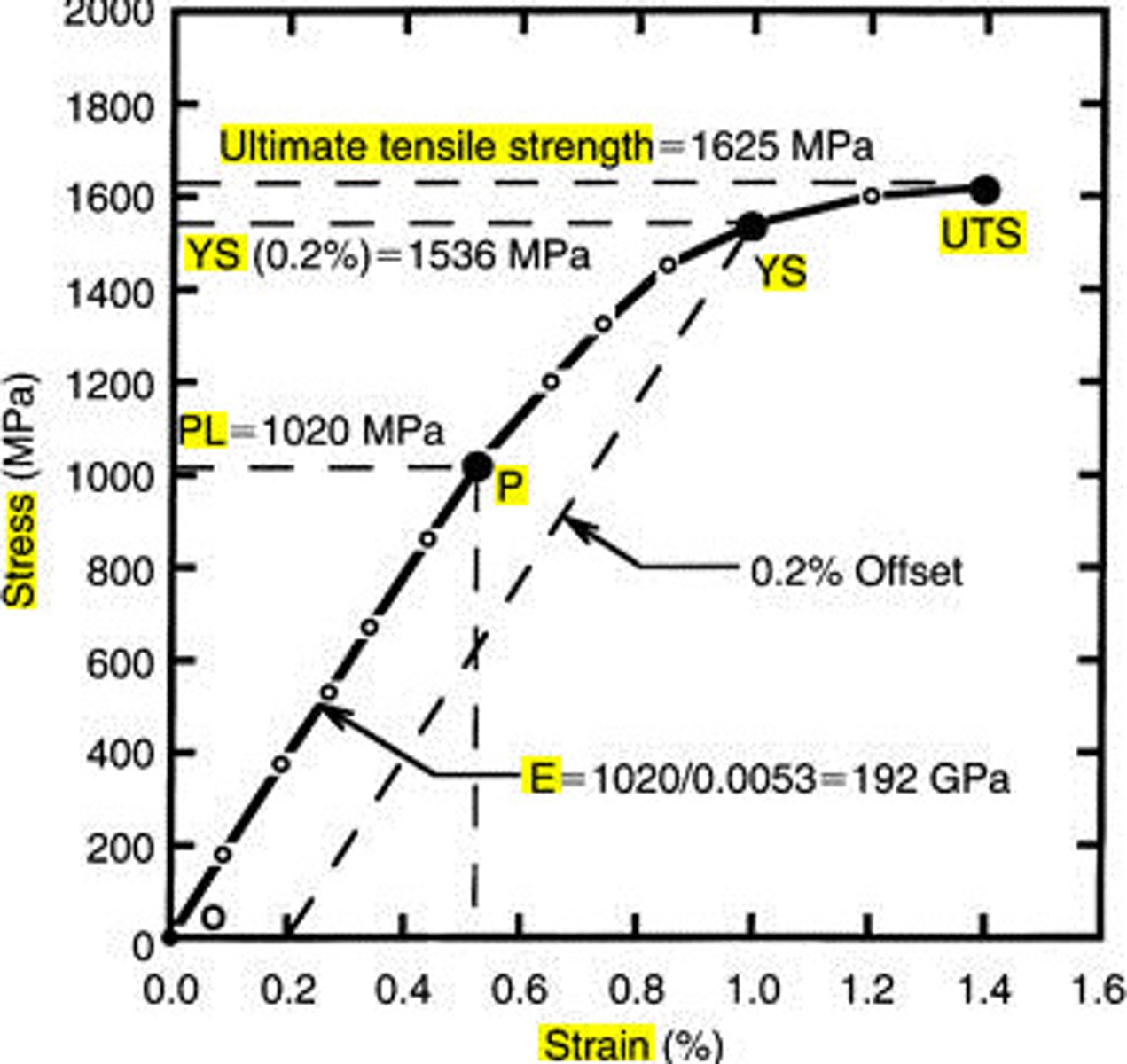
modulus of elasticity
a coefficient of elasticity of a material, expressing the ratio between a unit stress and the corresponding unit strain caused by the stress, as derived from Hooke's law and represented by the slope of the straight-line portion of the stress-strain diagram (coefficient of elasticity)

plastic deformation
Occurs when a material deforms, or changes shape, as a stress is applied and remains in the new shape when the stress is released.
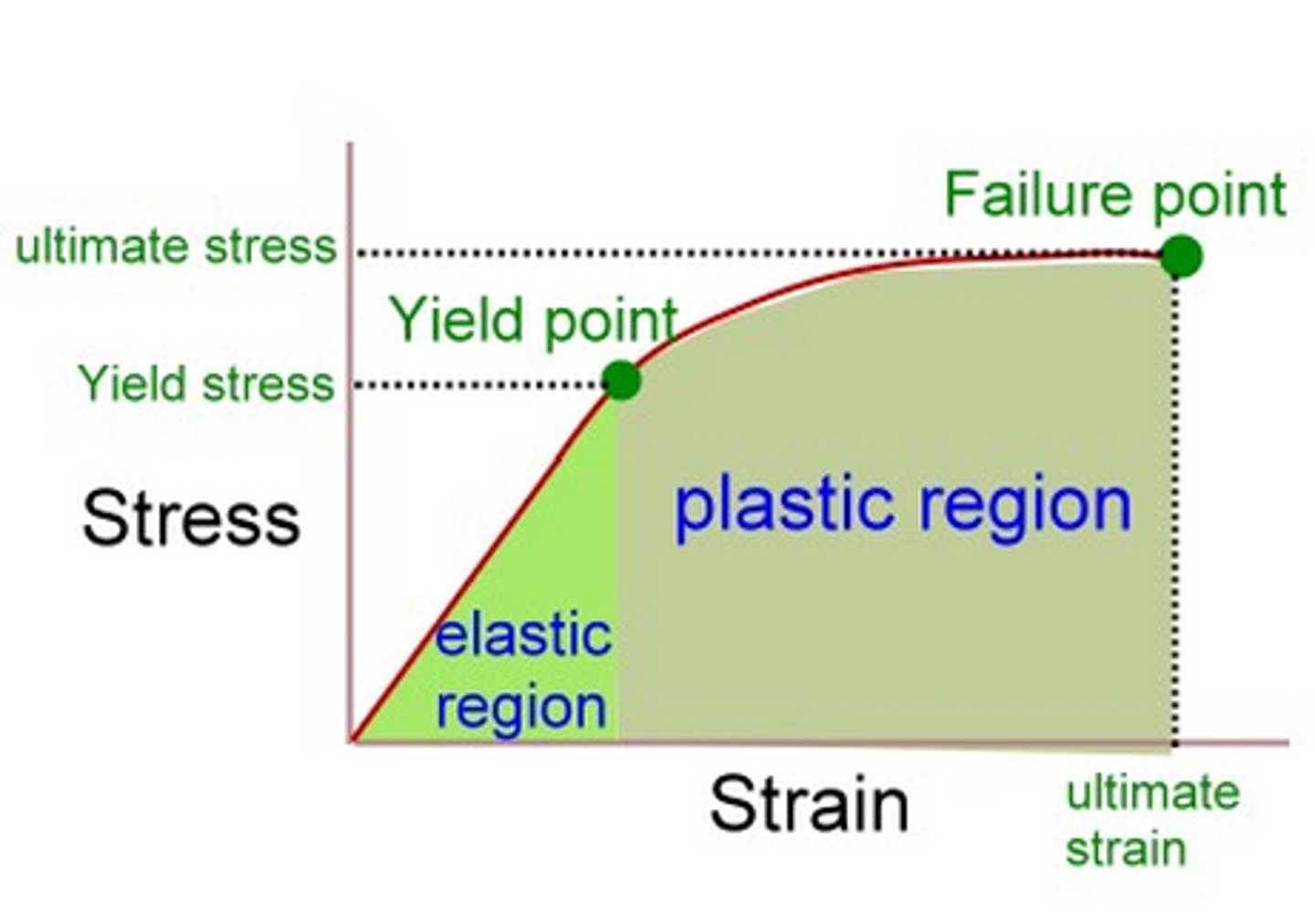
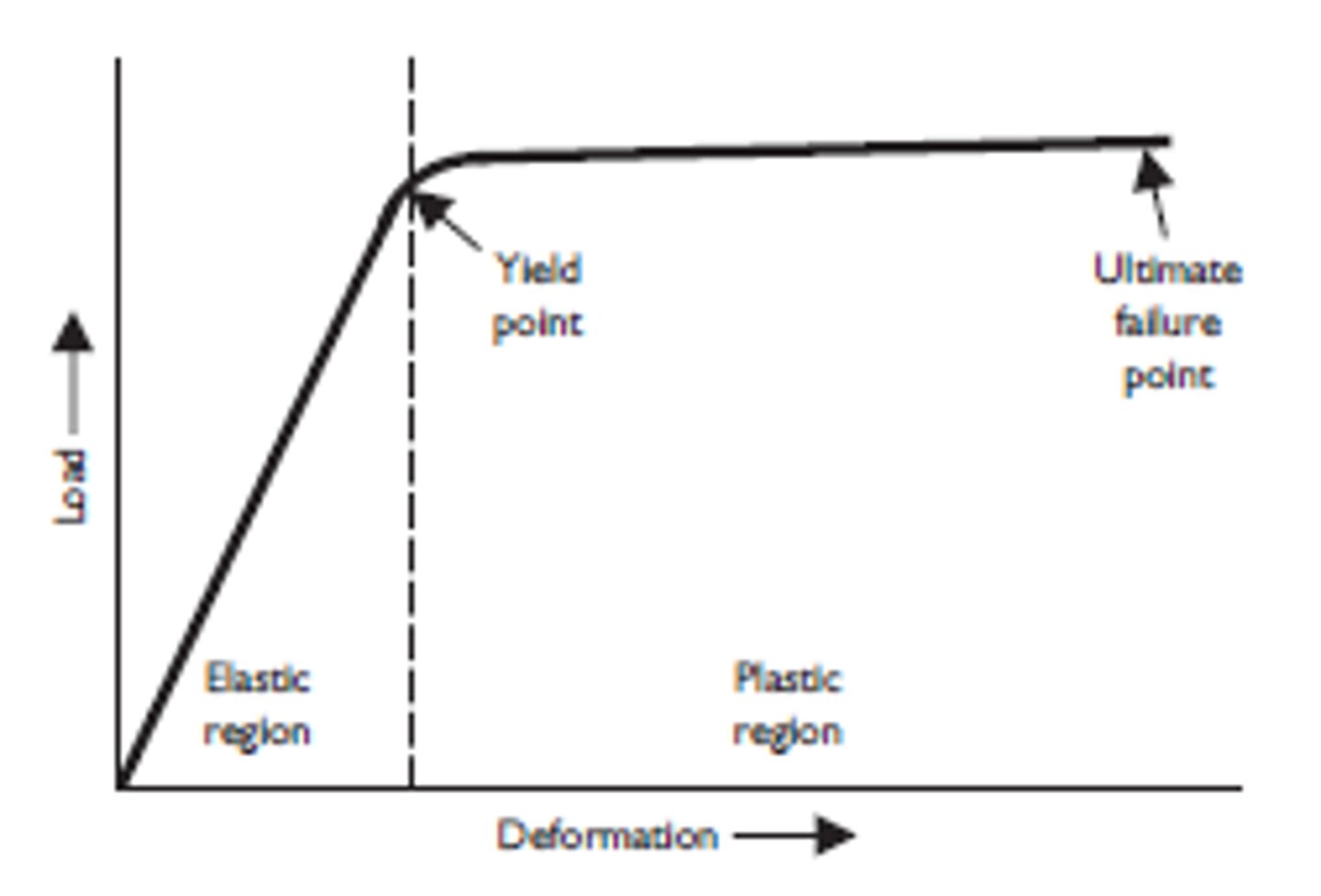
proportional limit
Point at which the deformation is no longer directly proportional to the applied force. Hooke's Law no longer applies
safe stress
A stress used for design purposes; for ductile metals, it is the yield strength divided by a factor of safety.
Ultimate tensile strength
Maximum stress which can be applied to a material before failure

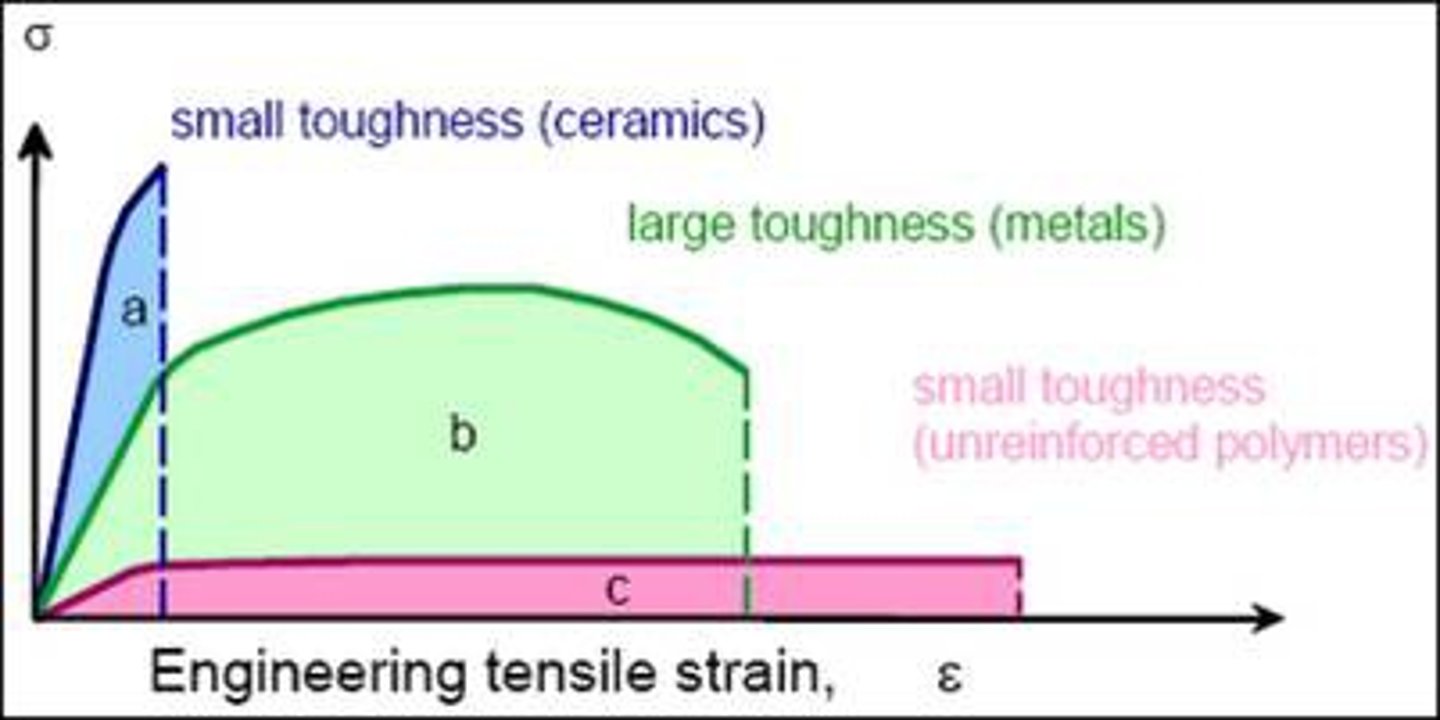
toughness
Mechanical property of a material that indicates the ability of the material to handle overloading before it fractures.
true strain
strain measure in which increments in strain are based on current dimensions of the structure
true stress
a ratio of the force applied to a sample and the instantaneous cross-sectional area of the sample
viscoelasticity
property deforms slowly with load and returns originial shape slowly with removal of load
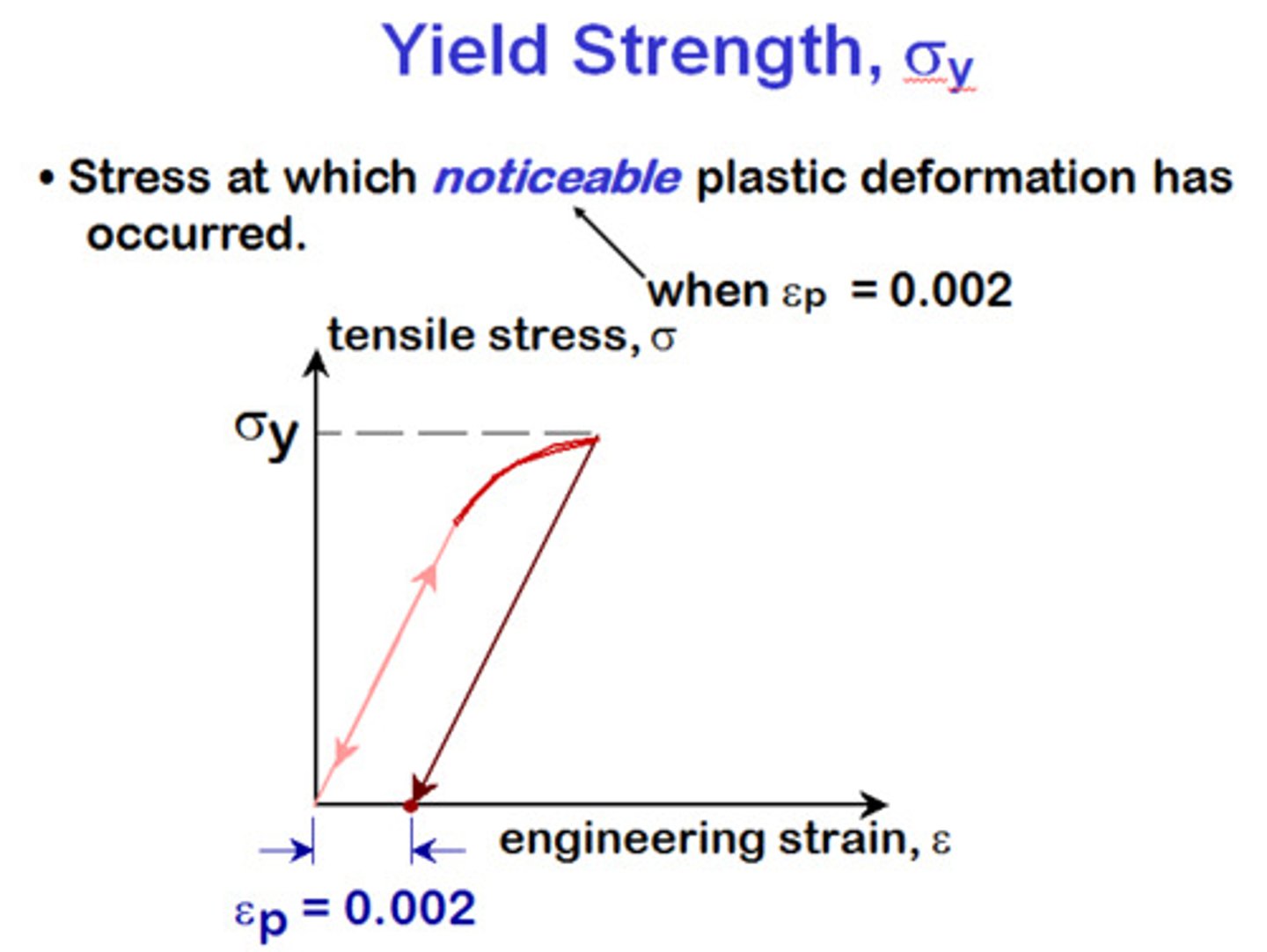
yield strength
point a which, when exceeded, a material will no longer completely return to its original shape after removing the applied load
cold working
permanent deformation of metals and alloys below the temperature at which a strain-free microstructure is produced continuously
critical resolved shear stress
Stress required to cause slip in pure metal single crystal. Depends on crystal structure, atomic bonding characteristics, temp, orientation of slip plane
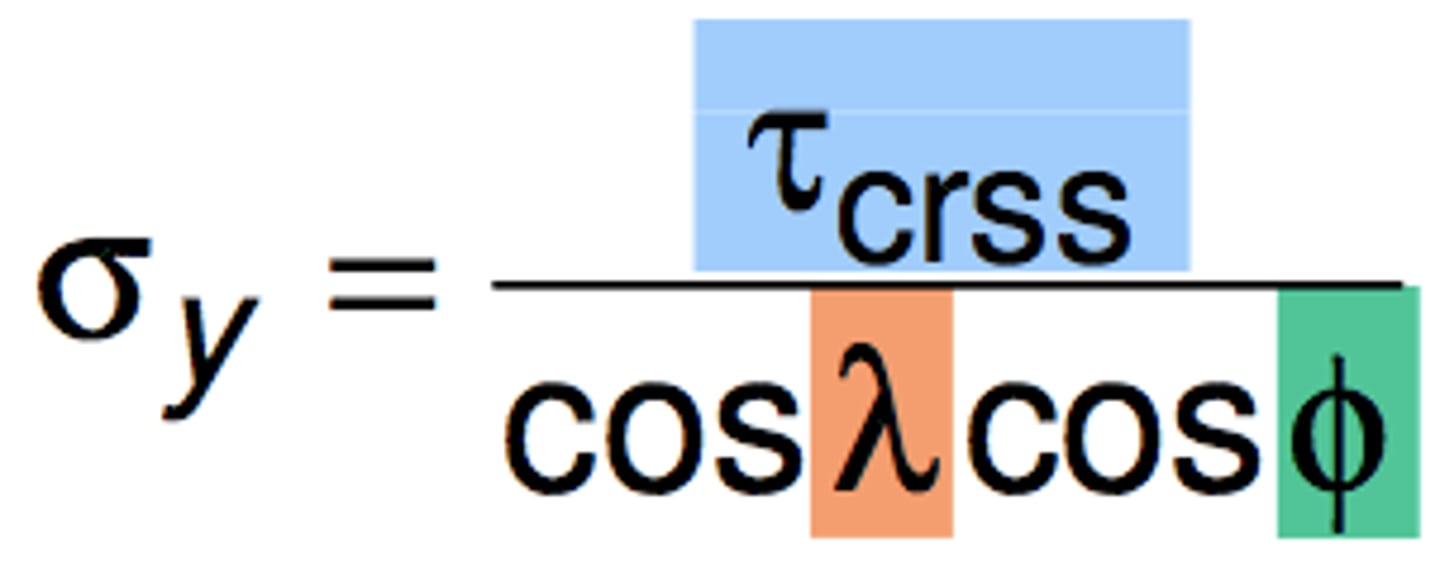
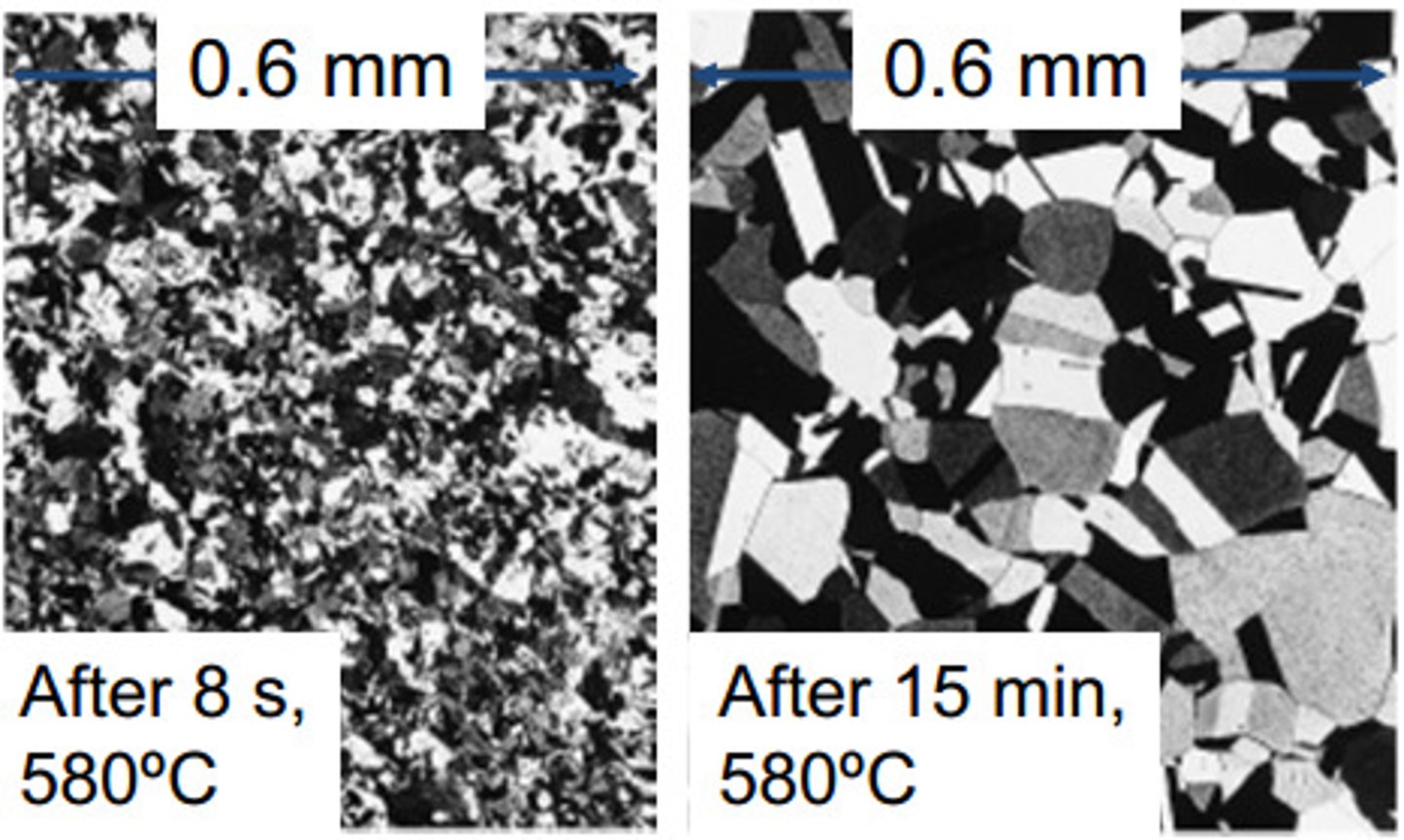
grain growth
the third step in the formation of crystallites, which is dependent on temperature and can be described using the arrhenius equation