Balancing equations
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
18 Terms
What are the five types of chemical reactions?
Synthesis, decomposition, combustion, singular replacement, and double replacement
Synthesis
A+B —> AB
Decomposition
AB —> A+B
Combustion
C x Hy + O2 —> CO2 + H2O
Single replacement
A + BC —> B + AC
Double replacement
AB + CD —> AD + BC
Indicators of a chemical change
-change in color
-production of gas
-formation or precipitate
-temperature
-light
Qualitative
Observation that’s a color, smell, taste, texture
Quantitative
Observation based on numbers
What comes before the —>
Reactants
What comes after the —>
Products
A chemical equation is balanced when…
The number of atoms of each element is the same in both reactants and products
In a chemical equation the coefficients…
Appear before the chemical formulas
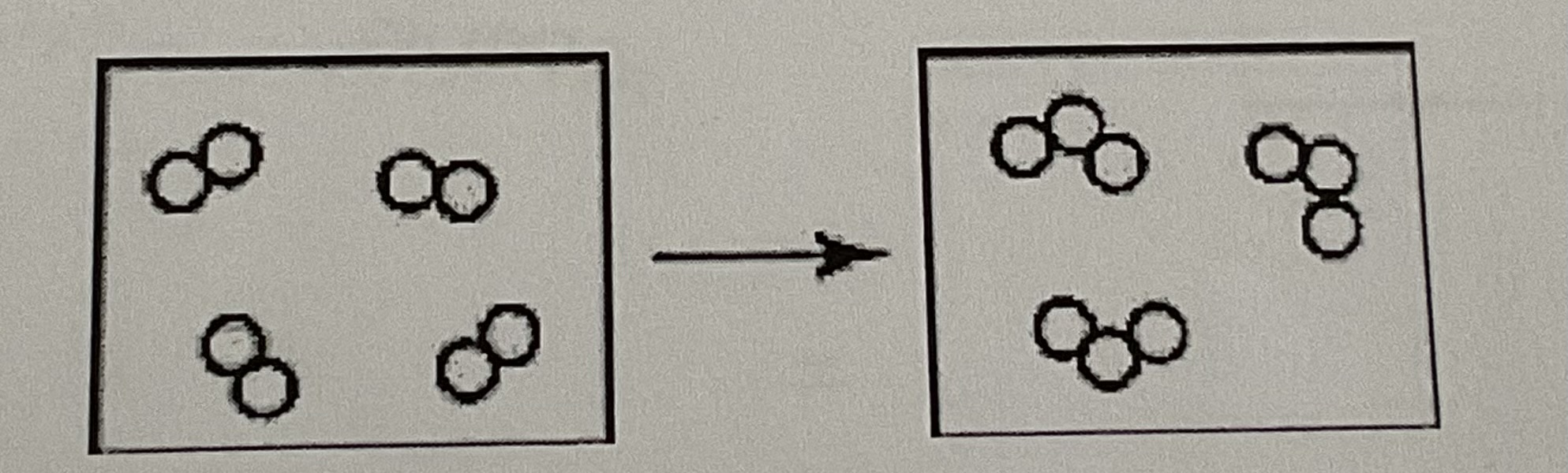
What is wrong with the following model of a chemical reaction?
One box contains more atoms than the other
What is a chemical reaction?
When one or more new compounds are formed by rearranging atoms
What is a chemical equation?
It is a shorthand notation for illustrating a chemical reaction
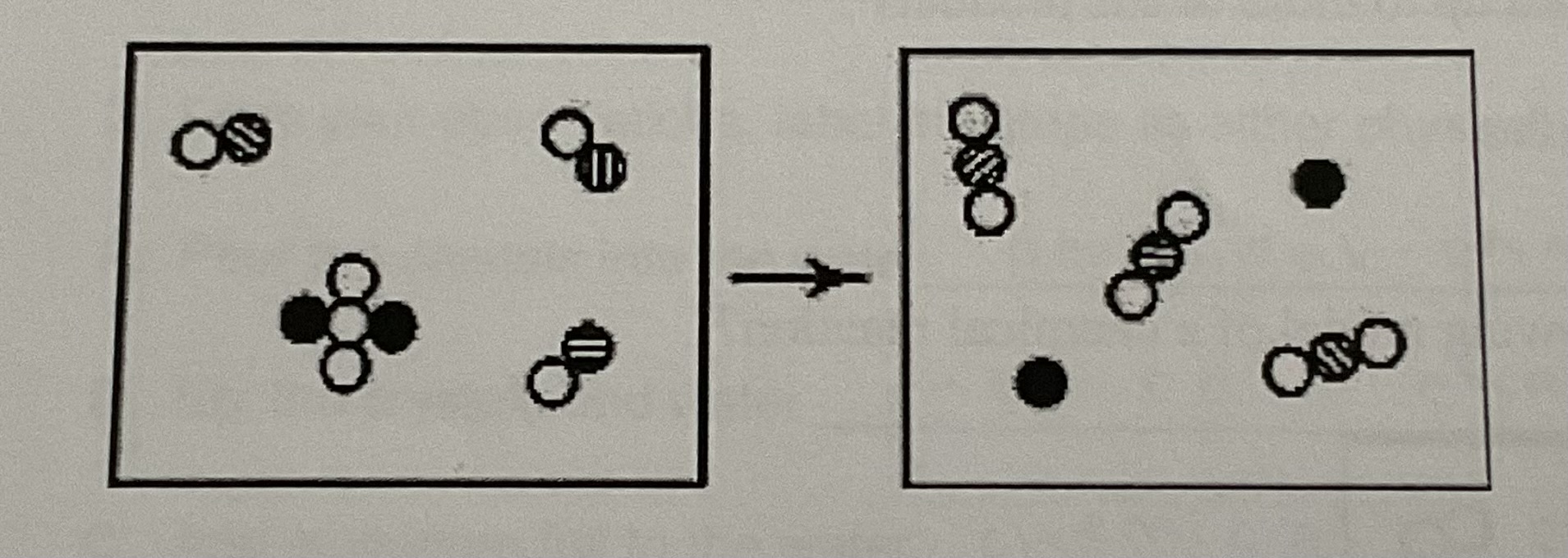
The reactants shown schematically below represent iron oxide, Fe2O3 and carbon monoxide, CO. Which of the following is the correct full balance chemical equation for what is depicted?
A) Fe2O3 + 3 CO —> 2 Fe + 3 CO2
B) Fe2O3 + 3 CO —> 3 FeO + 2 C
C) Fe2O3 + 3 CO —> 3 FeO2 + 2 C
D) Fe2O3 + 3 CO —> 2 Fe + 3 C2 O
A) Fe2O3 + 3 CO —> 2 Fe + 3 CO2
What is a physical change?
State of matter changes, but the molecular structure states the same