Comprehensive Biology: Cell Metabolism, Photosynthesis, and Cellular Respiration
1/99
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
100 Terms
Cellular respiration
the process by which cells convert energy stored in glucose into adenosine triphosphate (ATP), the main energy currency of the cell
Metabolism
The sum of all reactions. All the chemical reactions that occur within the body to maintain life and function. Transforms matter and energy.
Metabolic Pathways
When a specific molecule ends with a product, each step is catalyzed by a specific enzyme.
Enzyme
Protein molecule that speeds up reactions.
Catabolic
Release energy and break down complex molecules.
Anabolic
Consume energy and build up complex molecules.
Bioenergetics
The study of how energy flows through living organisms.
Energy
The capacity to cause change. Energy can also be used to do work. Work is the movement of matter against opposing forces.
Kinetic Energy
energy associated with motion.
Thermal energy
this is kinetic energy associated with random movement of atoms or molecules, This energy is measured by temperature.
Potential Energy
the stored energy in an object due to its position, state, or arrangement.
Chemical Energy
potential energy available for release in a chemical reaction.
Thermodynamics
The study of energy transformations. The relationship between heat, energy, and work, and how they are converted and transferred.
Isolated system
System unable to exchange energy or matter with its surroundings.
Open system
Systems where energy can be transferred between the system and its surroundings.
Closed System
Systems where only energy can be transferred. Everything that is happening is only happening in that system.
First Law of Thermodynamics
The energy of the universe is constant. Energy can be transferred and transformed, but it cannot be created or destroyed. Known as the principle conservation of energy.
Second Law of Thermodynamics
Energy is lost as heat. Every energy transfer or transformation increases the entropy of the universe.
Entropy
The measure of molecular disorder or randomness.
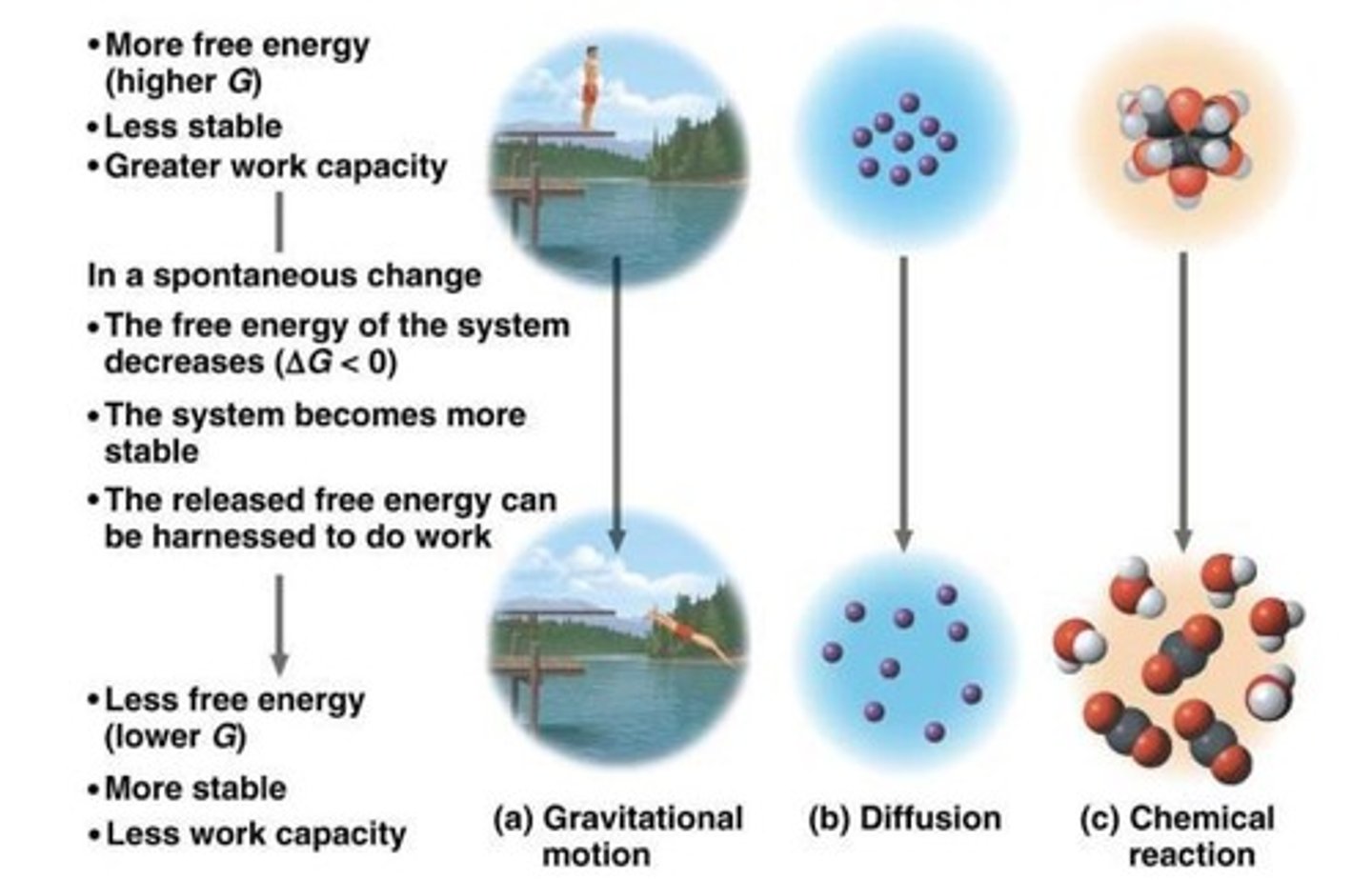
Spontaneous Process
occur without energy input, they can occur quickly or slowly (increase entropy).
Nonspontaneous Process
occur only if energy is provided (decrease entropy).
Enthalpy
The sum of its internal energy and the product of its pressure and volume.
Free energy
Energy that can do work when temperature and pressure are uniform throughout the system, as in a living cell.
Exergonic
Net release of free energy, and spontaneous. Delta G is negative.
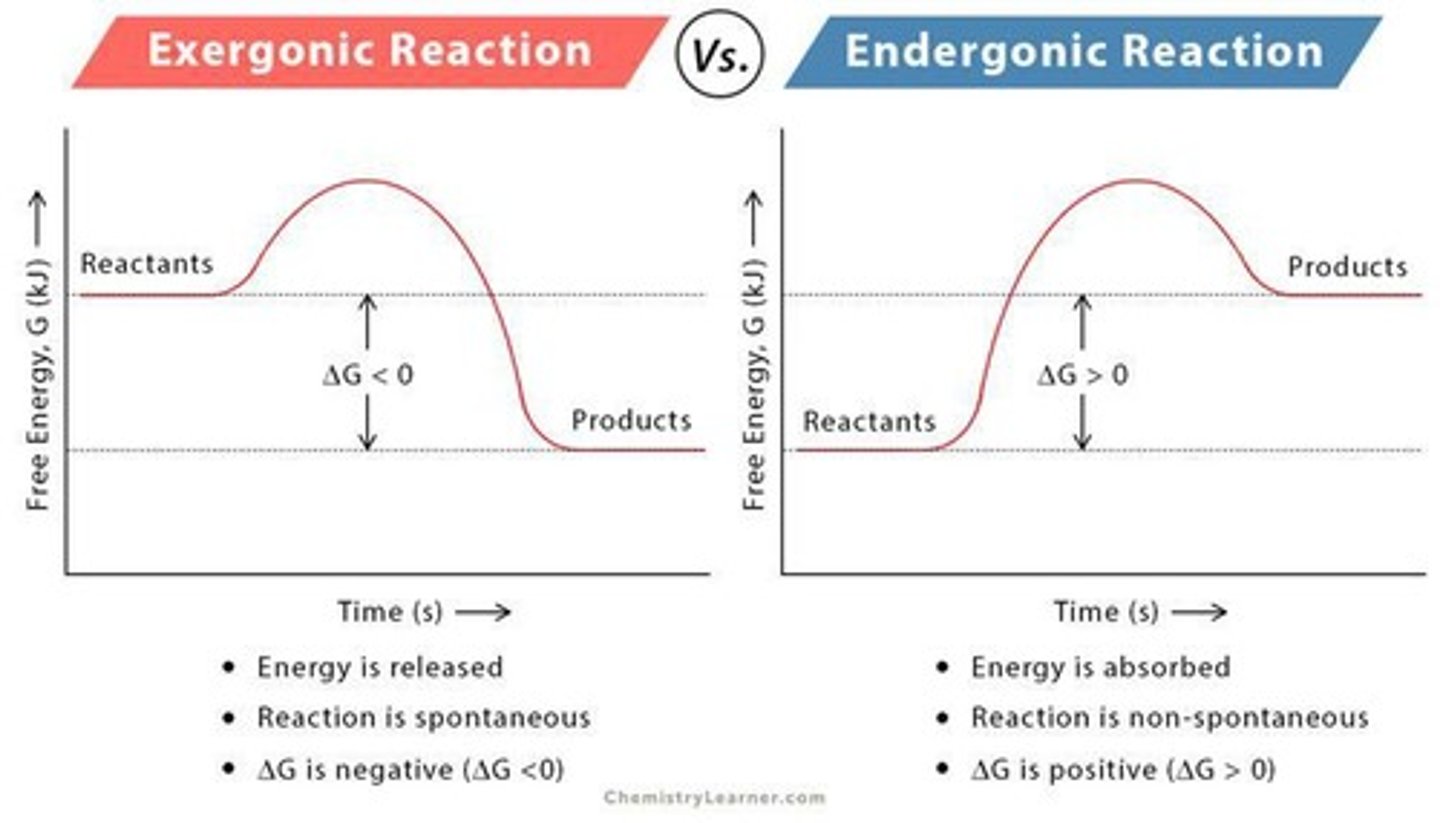
Endergonic
Absorbs free energy from its surroundings. Delta G is positive.
Equilibrium
A state of balance. The forward and reverse reactions occur at the same rate.
Equilibrium in Closed Systems
Reactions in a closed system eventually reach equilibrium and then can do no work.
Equilibrium and Work
Things at equilibrium cannot do work because work requires an imbalance of forces or energy to cause a change in motion or state.
Open Systems
Reactions in an open system are not at equilibrium; they have a constant flow of materials in and out.
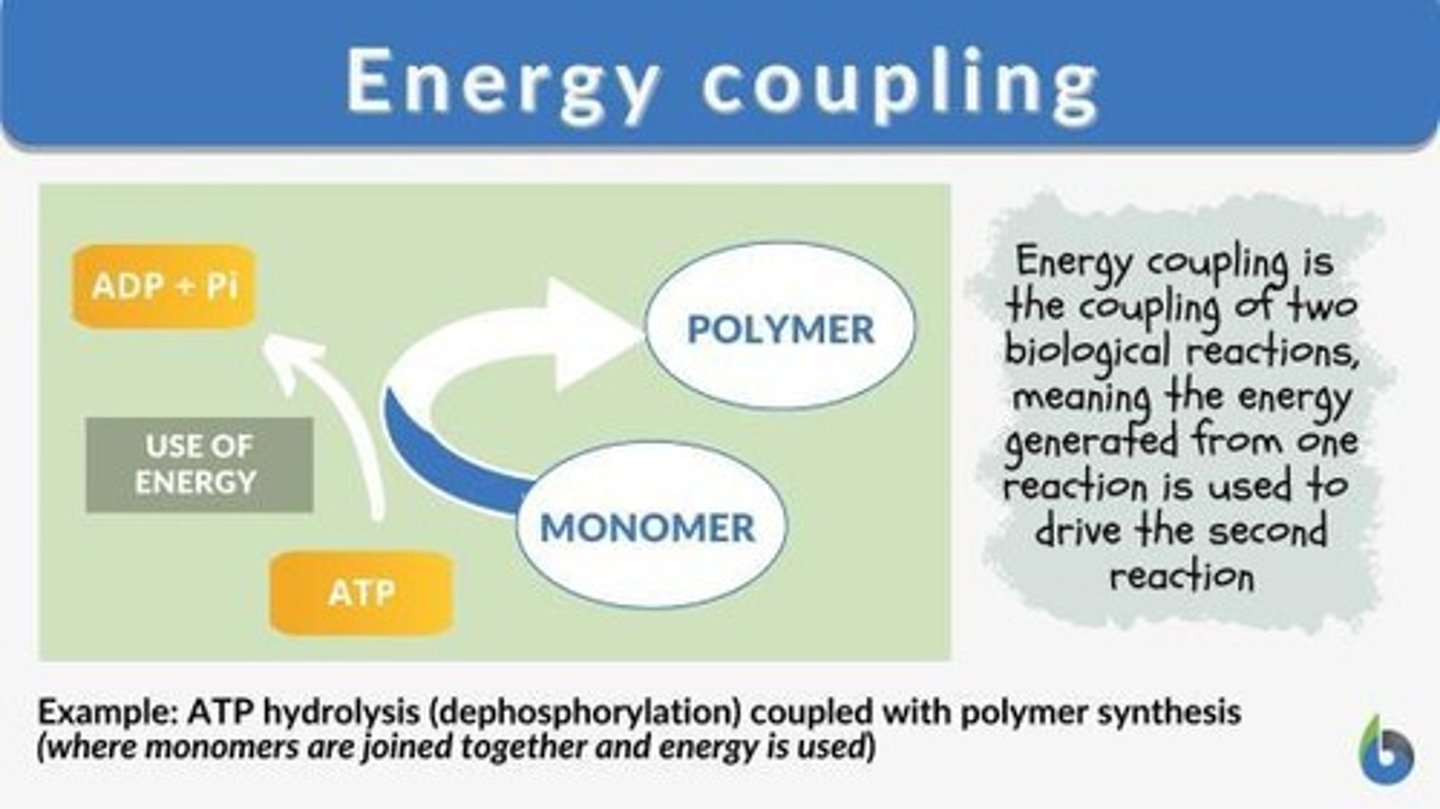
Energy Coupling
The use of an exergonic process to drive an endergonic one. Most energy coupling in cells is mediated by ATP.
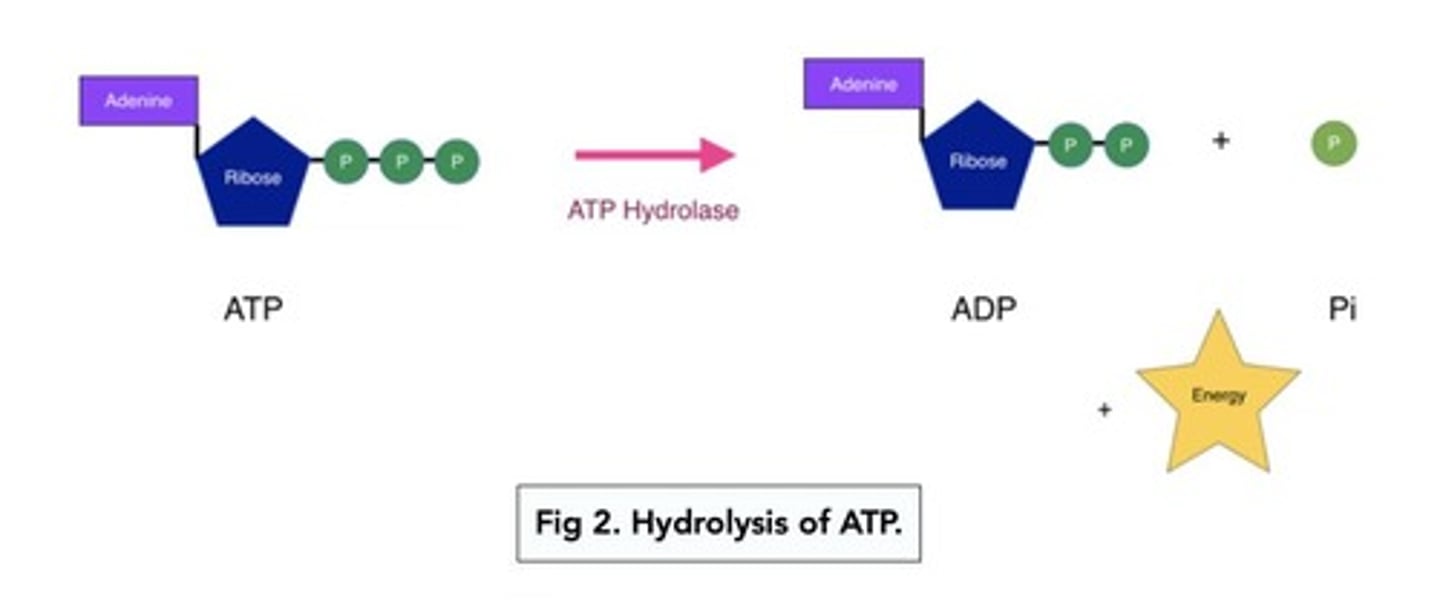
ATP Hydrolysis
ATP hydrolysis is the process where ATP (adenosine triphosphate) is broken down by a water molecule to release energy.
Chemical Work
Pushing endergonic reactions.
Transport Work
Pumping substances against the direction of spontaneous movement.
Mechanical Work
The energy transferred to or from an object by a force that causes displacement, such as contraction of muscle cells or beating cilia.
ATP
ATP is a renewable resource that is regenerated by the addition of a phosphate group to ADP.
Regeneration of ATP
ATP is a renewable resource that is generated by addition of a phosphate group to ADP. Free energy needed to phosphorylate ADP comes from catabolic reactions in the cell.
Enzymes
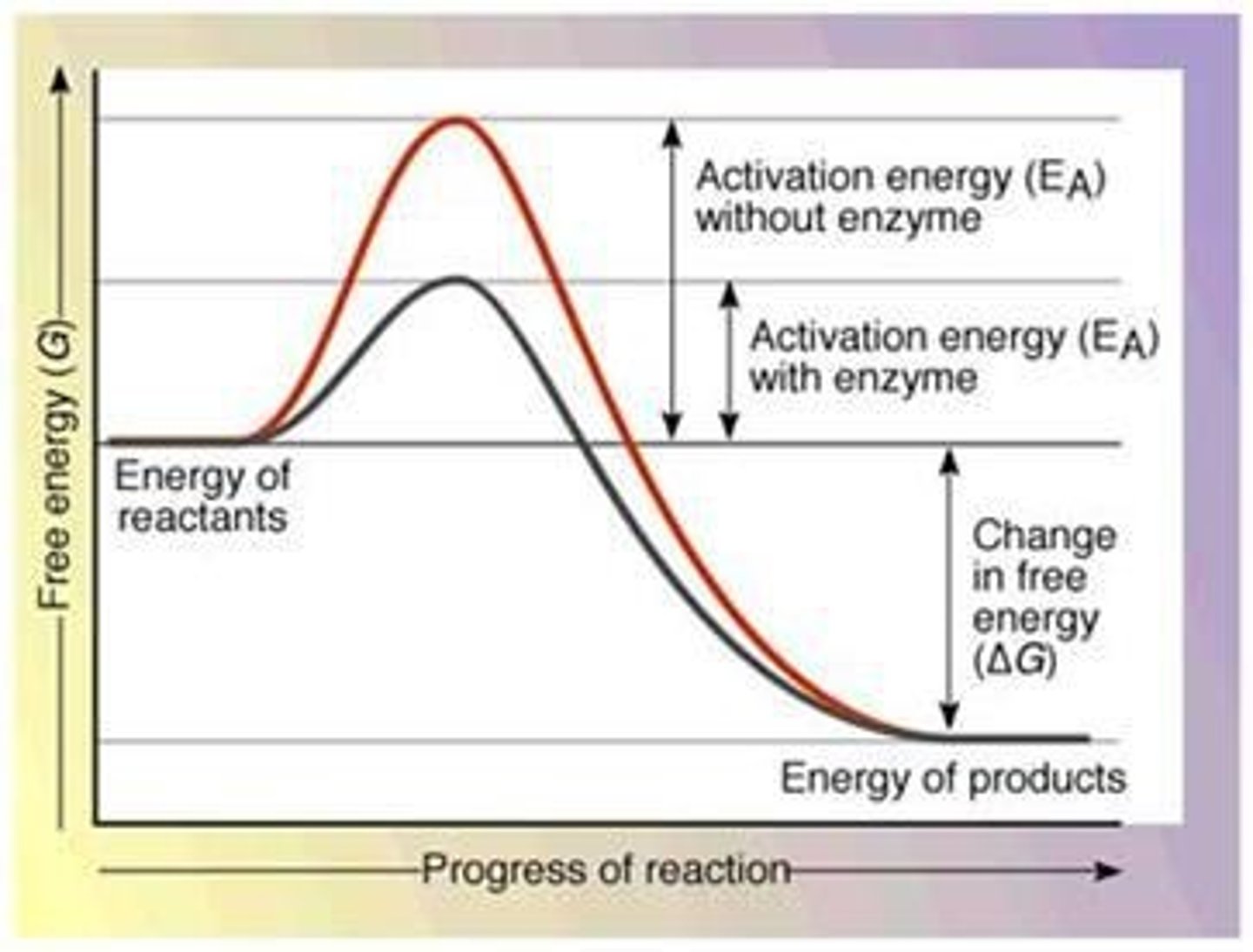
Speed up metabolic reactions by lowering energy barriers. Enzymes are catalytic proteins.
Catalyst
Chemical agent that speeds up a reaction without being consumed by the reaction; they use activation energy to speed up the reaction.
Activation Energy Barrier
The initial energy needed to start a chemical reaction is called the free energy of activation or activation energy.
Substrate Specificity to Enzymes
The reactant that binds to an enzyme is called a substrate. When they bind, this is called an enzyme-substrate complex and the substrate is converted to a product.
Induced Fit
When the active site of the enzyme adjusts to maximize the fit of the substrate.
Enzyme Saturation
An enzyme is saturated when many enzymes have their active sites engaged.
Factors Affecting Enzyme Activity
Enzyme activity can be affected by temperature, pH, cofactors, and inhibitors.
Cofactors
These are non-protein enzyme helpers that affect enzyme activity by either binding to the active site to help stabilize the enzyme-substrate complex or by participating directly in the reaction.
Cofactors Composition
Cofactors are made of inorganic substances, like metal ions, or organic molecules, which are often derived from vitamins.
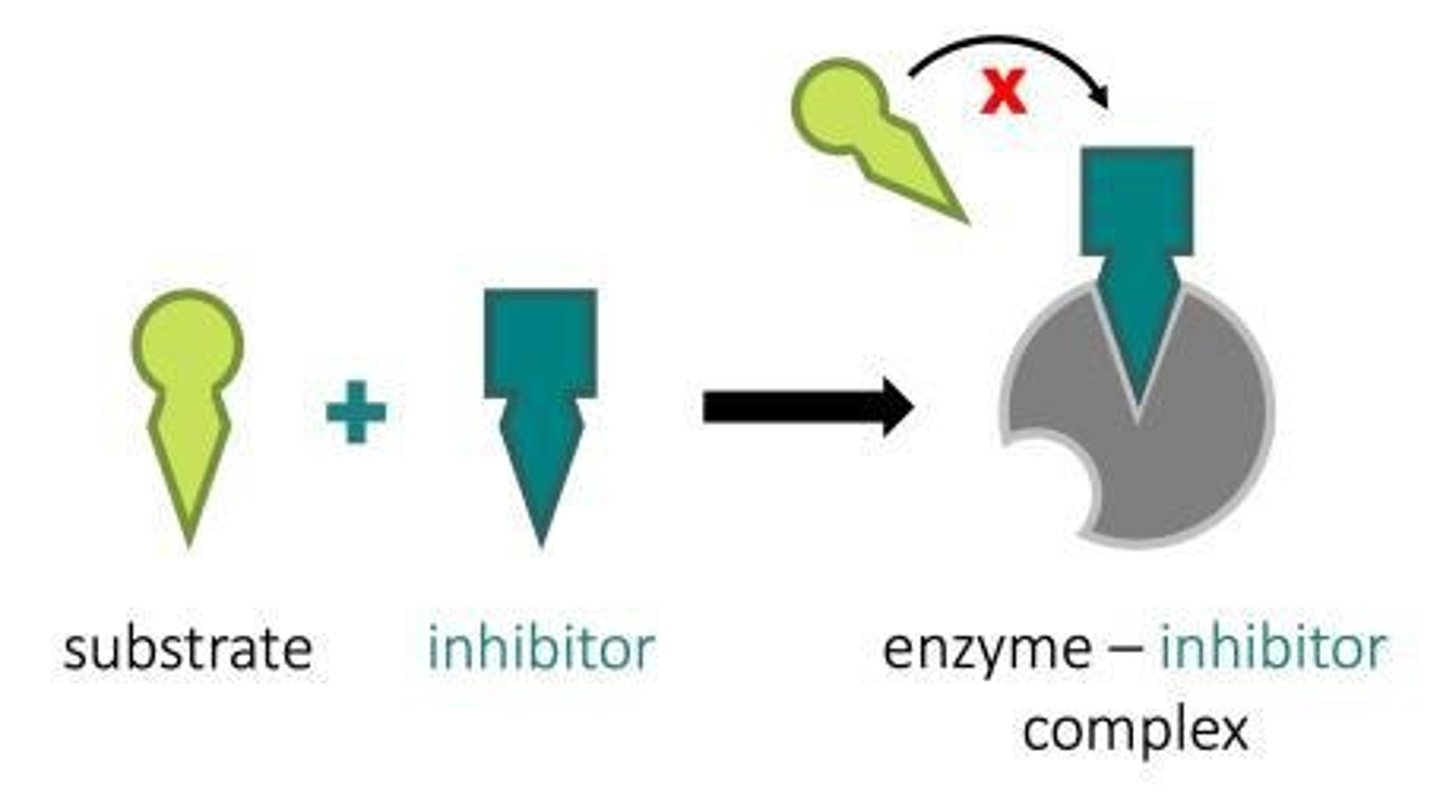
Competitive Inhibitor
bind to active site competing with substrate
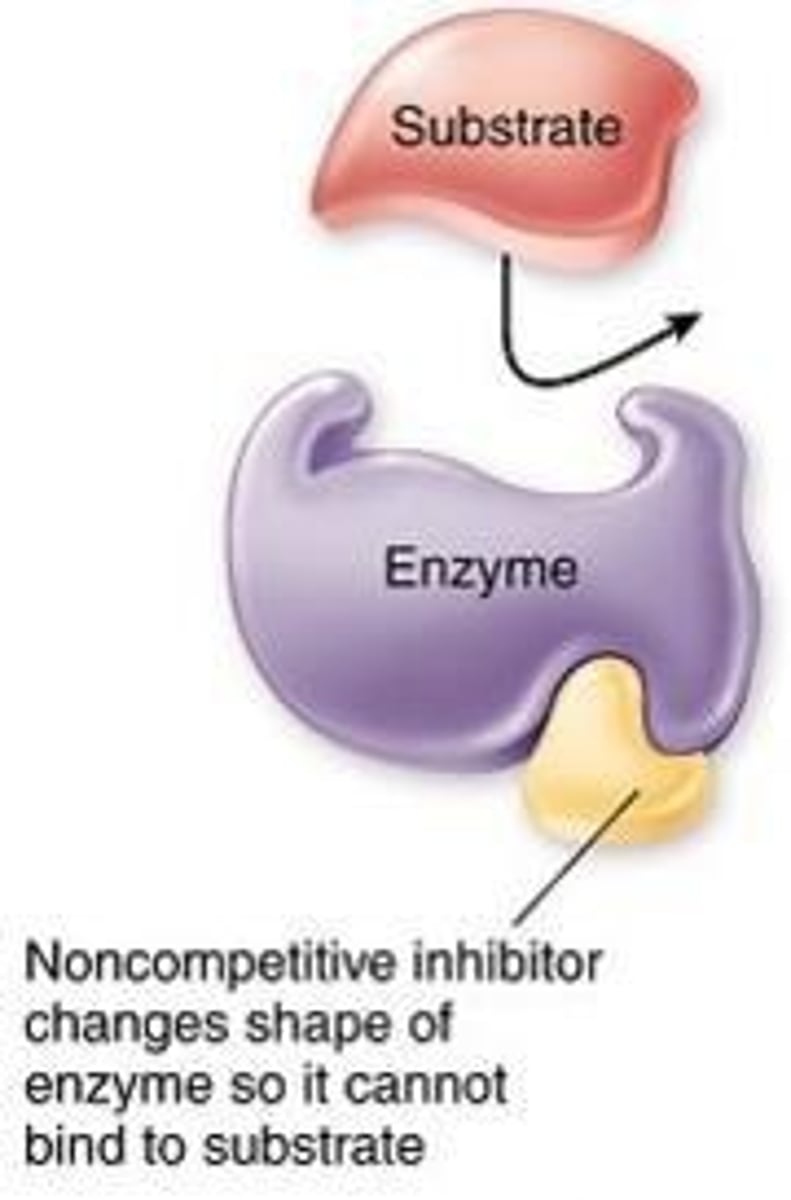
Noncompetitive Inhibitor
bind to another part of an enzyme, causing it to change shape making the active site less effective
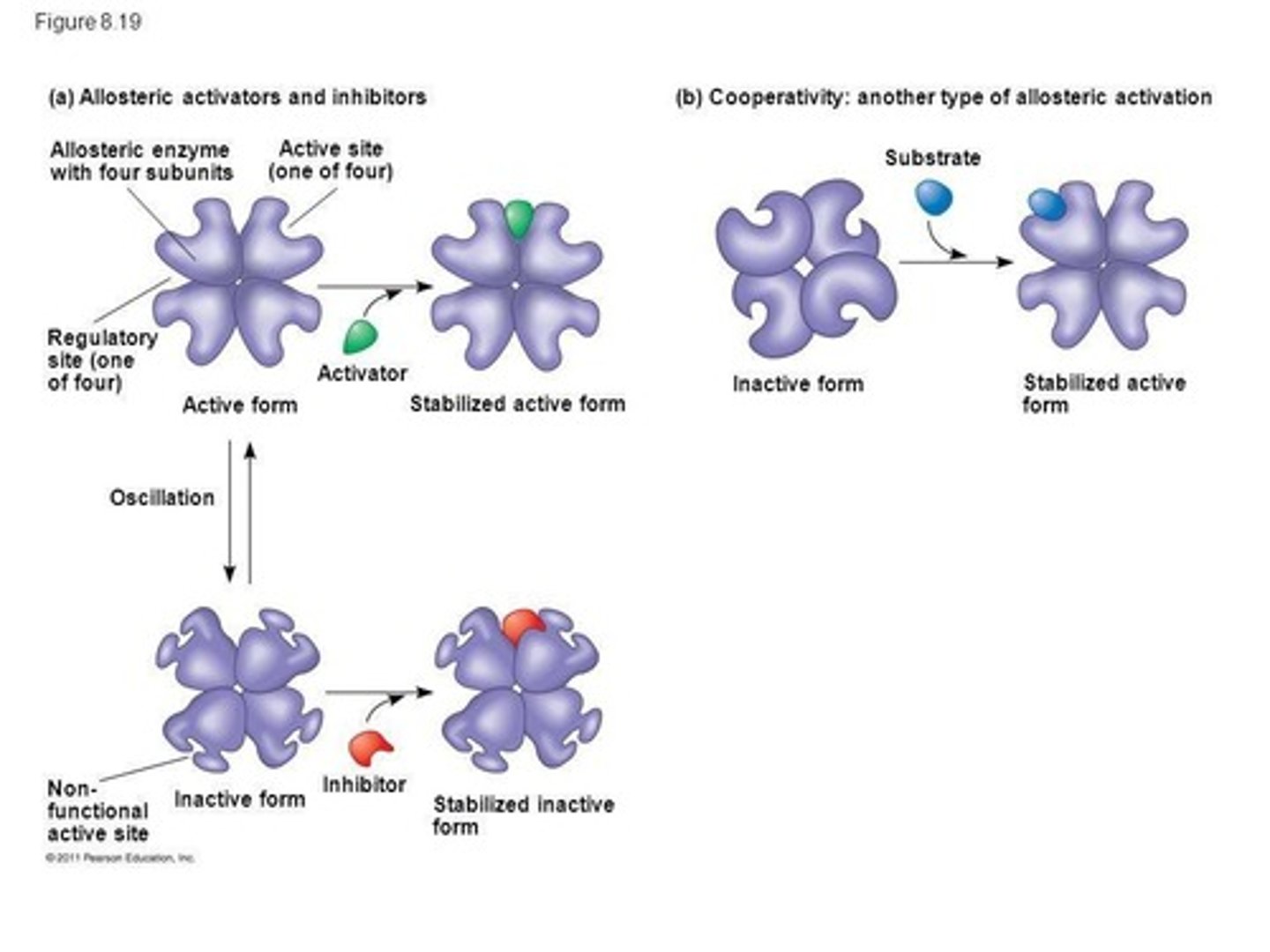
Allosteric Activation
occurs when a regulatory molecule binds to a protein at one site and affects the proteins function at another site. It has active and inactive forms. Cooperativity can amplify enzyme activity.
Feedback Inhibition
a metabolic process where the end product of a pathway inhibits an enzyme at the beginning of the same pathway, preventing overproduction and maintaining cellular balance
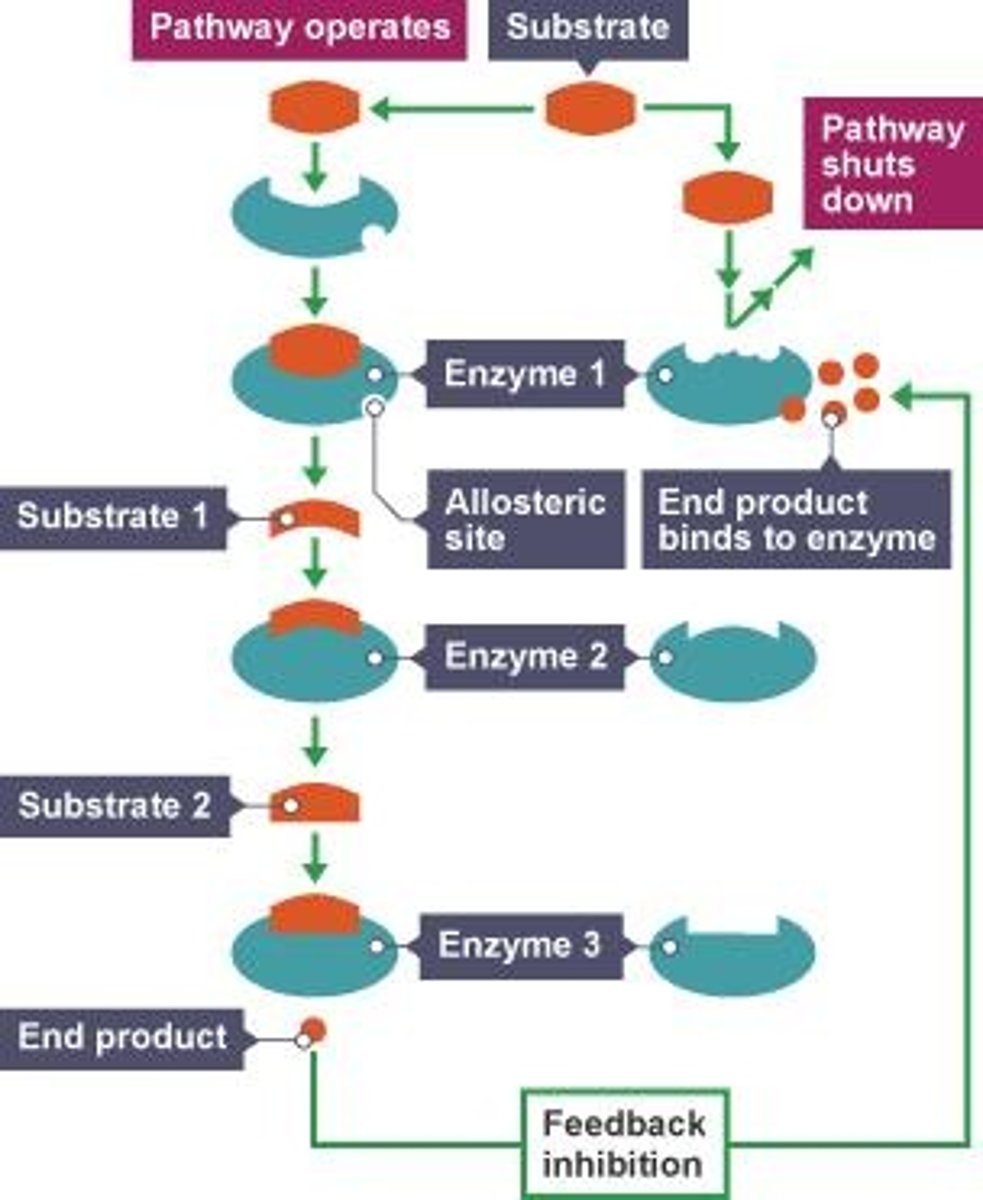
Localization
structures in the cell help bring order to metabolic pathways. The specific placement of enzymes within different cellular compartments, such as the cytoplasm, nucleus, mitochondria, and endoplasmic reticulum, which is crucial for efficient cellular function.
Energy flow in ecosystems
Energy flows into an ecosystem as sunlight and leaves as heat.
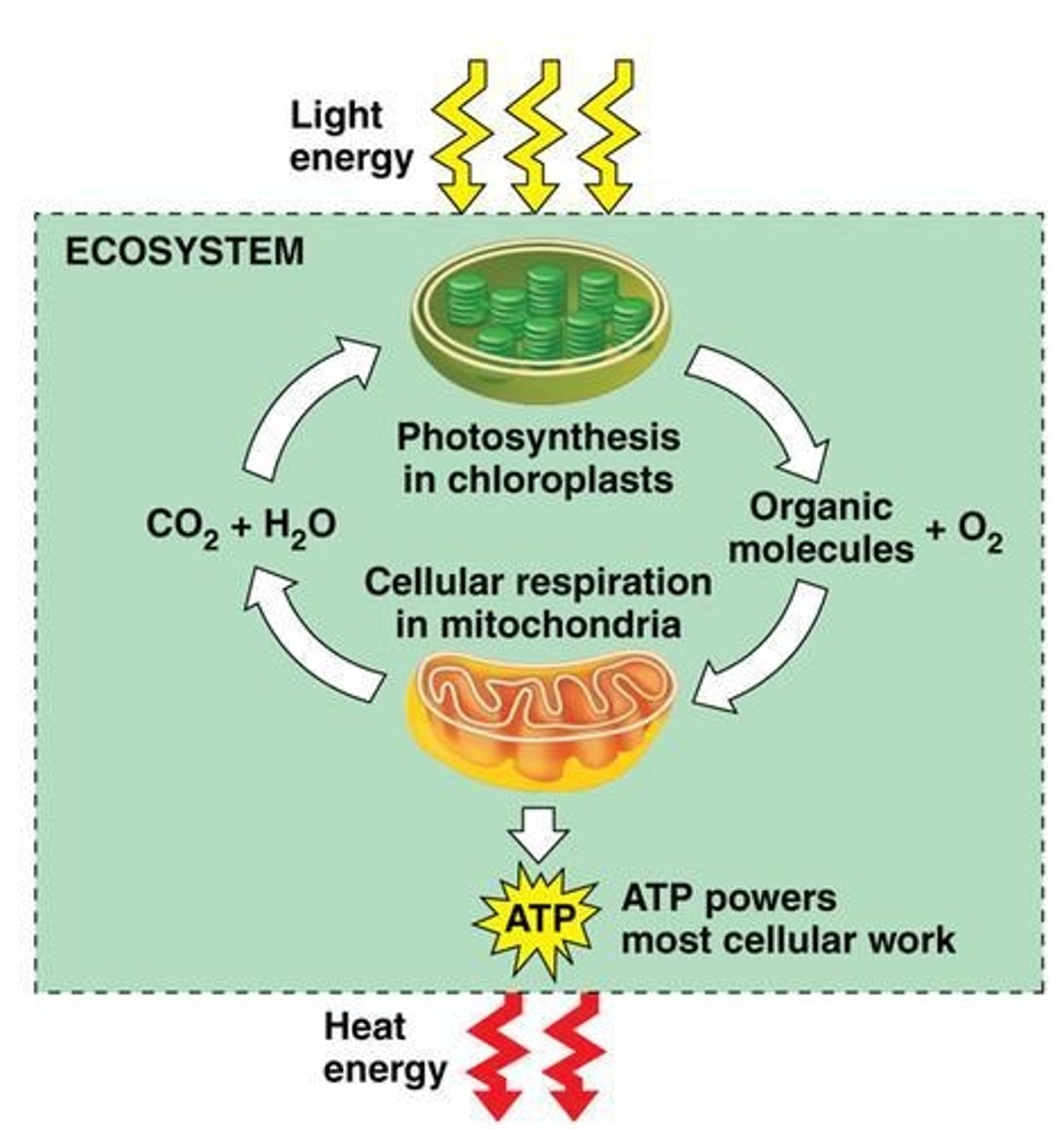
Photosynthesis
Input: Light energy, water, and carbon dioxide; Output: Organic molecules (Glucose) and oxygen.
Aerobic Respiration
Consumes organic molecules and O2 and yields ATP.
Anaerobic
Consumes compounds other than O2, produces ATP but in a smaller amount than in aerobic respiration.
Fermentation
A partial degradation of sugars that occurs without O2; Uses substrate-level phosphorylation to generate ATP.
Alcohol Fermentation
Pyruvate converted to ethanol.
Lactic Acid Fermentation
Pyruvate reduced by NADH to form lactate and NAD+.
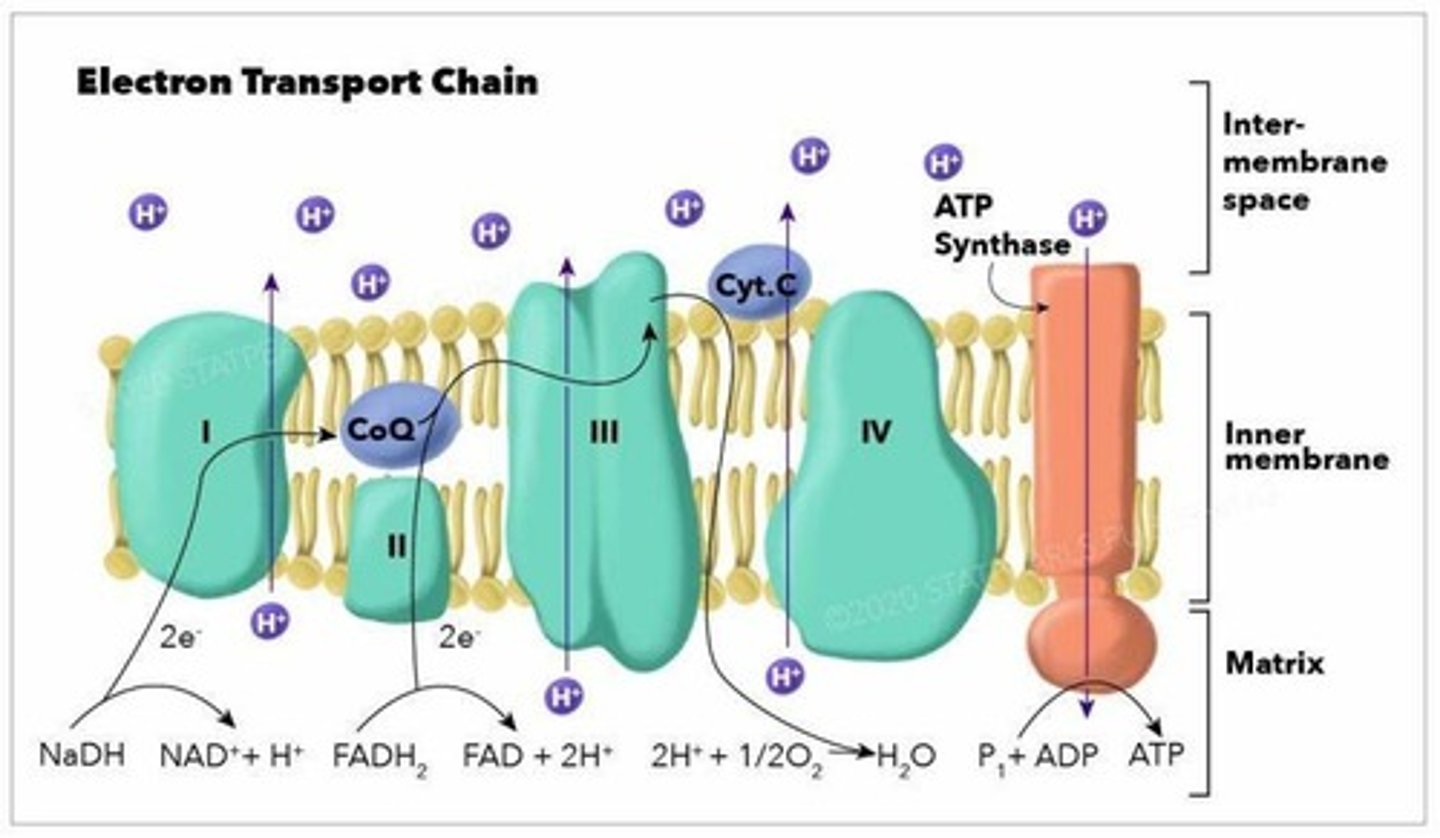
Electron Transport Chain
A series of protein complexes in the inner mitochondrial membrane that uses high-energy electrons from NADH and FADH2 to pump protons H+ from the mitochondrial matrix to the intermembrane space, creating a proton gradient.
Glycolysis
Input: one molecule of glucose, two molecules of ATP, and two molecules of NAD+; Output: 2 pyruvate molecules, a net gain of 2 ATP molecules, and 2 NADH molecules; Occurs in cytoplasm; Can occur with or without oxygen.
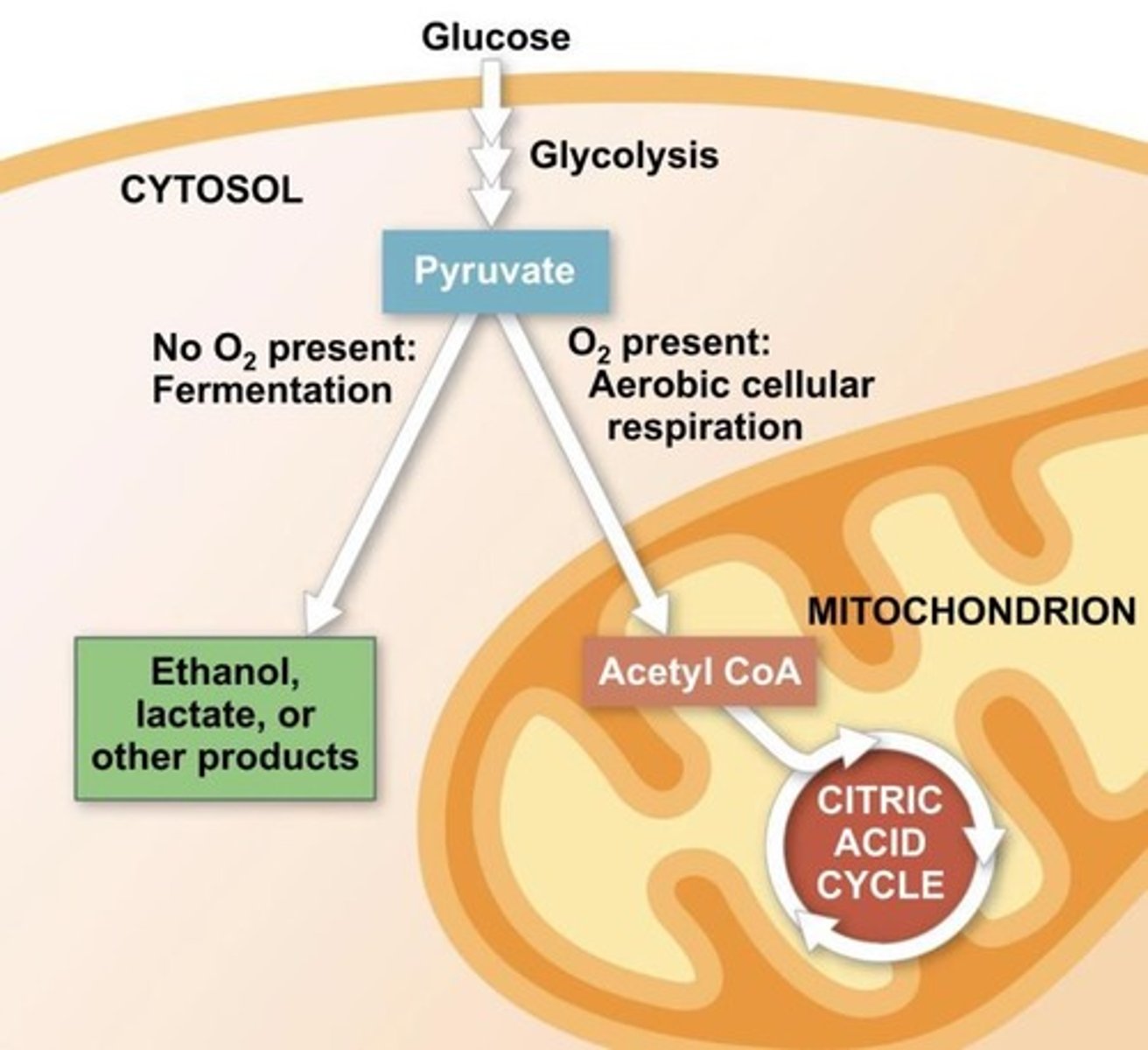
Pyruvate Oxidization
Input: pyruvate, Coenzyme A (CoA), and NAD+; Output: acetyl-CoA, NADH, and carbon dioxide.
Citric Acid Cycle
Input: acetyl-CoA, NAD+, 1 FAD, and 1 ATP; Output: 2 CO2, 3 NADH, 1 FADH2, and 2 ATP; Completes breakdown of pyruvate; Occurs in mitochondrial matrix.
Oxidative Phosphorylation
Input: oxygen, ADP, NADH, FADH; Output: 20-28 ATP, water, NAD+, FAD; Occurs in the inner mitochondrial membrane.
Chemiosmosis
Energy released as electrons passed down; Protein complex ATP synthase.
Obligate Anaerobes
Microorganisms that cannot survive in the presence of oxygen and require oxygen-free environments to live and grow.
Facultative Anaerobes
Yeast and bacteria that can survive using either fermentation or cellular respiration.
Autotrophs
Self feeders and producers of the biosphere; Produce organic molecules from inorganic sources.
Photoautotroph
Organisms that use energy from sunlight to make organic molecules.
Endergonic process
Photosynthesis is an endergonic reaction because it is a non-spontaneous process that requires a continuous input of energy from sunlight.
Redox reaction
A chemical reaction involving the transfer of electrons, where H2O is oxidized and CO2 is reduced during photosynthesis.
Heterotroph
Obtain organic material from other organisms
Chloroplasts
Typically in the leaf of plants; Found mainly in the cells of the mesophyll; Each mesophyll cell contains 30-40 chloroplasts; CO2 enters and O2 exists the leaf through microscopic pores called stromata; Stroma: envelope of two membranes surrounding dense fluid; Thylakoid: connected sacs in the chloroplast that compose a third membrane system, this is where the chlorophyll is located; Granum: stacks of thylakoids; Chlorophyll is the pigment that gives leaves their color.
Photosynthesis equation
The chemical change during photosynthesis is the reverse of the one that occurs during cellular respiration.
The splitting of water
Chloroplasts split H2O into hydrogen and oxygen.
Stages of Photosynthesis
1. Light Reactions: In thylakoids; Split H2O and release O2; Generate ATP from ADP by photophosphorylation; Reduce NADP+ to NADPH; Generate ATP and increase the potential energy of electrons by moving them from H2O to NADPH; Two possible routes of electron flow: Linear electron flow and Cyclic electron flow. 2. Calvin Cycle: In Stroma; Forms sugars from CO2 using ATP and NADPH; Begins with carbon fixation, incorporating CO2 into organic molecules; Uses ATP and NADPH to reduce CO2 to sugar; Anabolic, it builds sugar from smaller molecules by using ATP and the reducing power of electrons carried by NADPH; Carbon enters as CO2 and leaves as a sugar named glyceraldehyde 3-phosphate (G3P); There are three phases: 1. Carbon Fixation 2. Reduction 3.
Electromagnetic energy
Light is electromagnetic energy.
Electromagnetic radiation
Also called electromagnetic radiation.
Wavelength
Wavelength determines the type of electromagnetic energy.
Wavelength (definition)
Wavelength is the distance between crests of electromagnetic waves.
Photons
Light is discrete particles called photons.
Pigments
Pigments are substances that absorb visible light.
Spectrophotometer
Machine that measures pigments ability to absorb various wavelengths of light.
Absorption Spectrum
This is a graph plotting a pigment's light absorption versus wavelength.
Photosynthetic Pigments
Include Chlorophyll a, Chlorophyll b, and Carotenoids.
Chlorophyll a
(Violet-blue and red light work best for photosynthesis).
Chlorophyll b
(Broaden spectrum used for photosynthesis).
Carotenoids
(Broaden the spectrum of colors that drive photosynthesis).
Action Spectrum
A graph that shows the relative rate of photosynthesis at different wavelengths of light.
Excitation of Chlorophyll
When a pigment absorbs light, it goes from a ground state to an excited state, which is unstable.
Fluorescence
In isolation, some pigments also emit light, an afterglow called fluorescence.
Photosystem
A photosystem consists of a reaction-center complex surrounded by light-harvesting complexes.
Reaction-Center Complex
An association of proteins holding a special pair of chlorophyll a molecules and a primary electron acceptor.
Light-Harvesting Complex
Consists of pigment molecules bound to proteins.
Photosystems
Two types of photosystems: PS 2 can best absorb a wavelength of 680 nm; PS 1 can best absorb a wavelength of 700 nm.
Linear Electron Flow
Primary pathway in photosynthesis where electrons move from water to NADP+ through a series of two photosystems (PSII and PSI) in a single, unidirectional pathway.
NADP+
NADP+ reduces to NADPH. Electrons of NADPH are available for the reactions of the Calvin cycle.
Cyclic Electron Flow
Electrons cycle back from the Fd to the PS I reaction center via a plastocyanin molecule (Pc). Uses only photosystem I and produces ATP but not NADPH. No oxygen is released.
Chemiosmosis in Chloroplasts and Mitochondria
Transfer chemical energy from food to ATP. ATP is generated by chemiosmosis. Protons are pumped to the intermembrane space and drive ATP synthesis, as they diffuse back into the mitochondria matrix. Protons are pumped into the thylakoid space and drive ATP synthesis, and they diffuse back into the stroma.
Alternative Mechanisms of Carbon Fixation
On hot and dry days plants close stomata which conserves H2O but also limits photosynthesis. The closing of stomata reduces access to CO2 and causes O2 to build up. These conditions favor a wasteful process called photorespiration.
C3 Plants
Use the Calvin cycle to fixation of CO2, by rubisco produces a three-carbon sugar, 3-PGA, as the first step in photosynthesis.
Photorespiration
Rubisco adds O2 instead of CO2 in the Calvin cycle producing a two-carbon compound. Is considered wasteful because it consumes energy in the form of ATP and reducing power, releases previously fixed carbon and decreases overall sugar synthesis. Consumes O2 and releases CO2 without producing ATP or sugar.