Saponification
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
Identify the reactants used in the preparation of soap
Fat / lard / oil //
sodium hydroxide
Identify the solvent used in the preparation of soap
Ethanol
Name the co-product formed when soap is prepared in this way.
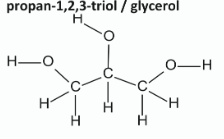
Draw the molecular structure of this co-product, including all atoms and bonds
Glycerol
Why was the reaction mixture refluxed?
Speeds up reaction / helps mix reactants / helps reaction reach activation energy
Suggest a suitable heating method for refluxing the reaction mixture and then distilling off the ethanol
Water bath (heated with bunsen burner or hot plate)
Justify the suitability of your suggested heating method
Oil (water) bath: gentle heating possible temperature easily controlled safe (no flame)
Heating mantle: temperature easily controlled / safe (no flame) / convenient / quick / easy to use
Bunsen with wire gauze: gentle heating possible / temperature easily controlled / flask not heated directly
Why is it desirable to remove all the ethanol?
Improves yield / to get more soap / makes soap less soluble in brine / helps precipitate soap / for re-use
Name the co-product of the reaction
Glycerol
Write the systematic IUPAC name for glycerol
1,2,3-propanetriol
What is brine?
Saturated solution of salt in water / salt water
Explain the function of the brine in the procedure
To precipitate soap / to dissolve components other than soap
Describe how the solid soap was separated from the solution in the beaker
Filter (filtration) / decant (pour) off brine (liquid)
Where is the excess NaOH at this stage of the procedure?
In brine / in the filtration flask / some mixed with soap
The diagram represents the arrangement of a number of soap molecules in water.
Explain why one end of a soap molecule is described as water loving (hydrophilic).
One end ionic (polar)
Calculation 2021 Q2
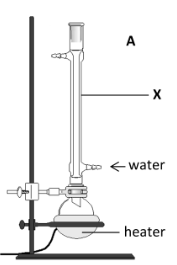
Name the piece of glassware labelled X
Condenser
Identify the two reactants and the solvent initially present in the flask
Reactants: fat // sodium hydroxide
Solvent: ethanol
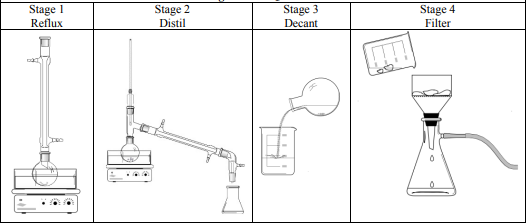
Name the type of reaction that occurred in stage 1 of the reaction
Saponification
What substance was removed by distillation in Stage 2?
Ethanol
Why was it necessary to wash the soap thoroughly in Stage 4?
To remove sodium hydroxide
How should the student have washed the soap?
Brine / ice-cold water
Calculation 2014 Q2
Suggest, with reference to its structure, how a soap like sodium stearate can dissolve both the non-polar oils and the ionic salts in sweat from the skin
Hydrocarbon part (end) is non-polar (hydrophobic) and dissolves oils (non-polar substances)
Ionic (hydrophilic) part (end) attracted to (dissolves) salts in sweat
Draw a fully labelled diagram of the reflux apparatus used in an experiment from your course where you refluxed a mixture
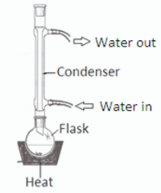
What happened to the liquid in the flask during reflux?
Hot vapour rose / liquid evaporated //
vapour was condensed / returned to flask
How did refluxing this mixture help bring the reaction to completion?
Allowed enough time / provide the activation energy / heating without loss of reactants
What was the purpose of the ethanol?
Solvent
Describe, with the aid of a labelled diagram, how the ethanol was removed after the reflux stage
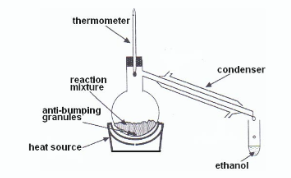
Why was a minimum of hot water used to dissolve the residue from the distillation?
To minimize soap remaining dissolved / maximise soap precipitating out
How was the soap isolated from the other substances left in the reaction mixture?
Added to brine
precipitated soap got by filtration
After isolating the soap, how was it purified and dried?
Purified: wash with brine / wash with water
Dried: warm place / oven
Calculation 2010 Q2
What would you observe, upon shaking, if a little of the soap prepared in this experiment is added to
i) a test tube containing deionised water,
ii) a test tube containing mineral water from a limestone region
i) (immediate) lather
ii) scum / no lather / less lather / does not easily form lather
What is the principle chemical difference between vegetable and animal fats?
Animal saturates //
vegetable unsaturated
Have different degree of saturation