BISC 1111 Group Activity 4
1/76
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
77 Terms
Membrane potential
the voltage across a membrane
voltage is created by differences in the distribution of positive and negative ions across a membrane
the inside of the cell is negative in charge relative to the outside, favoring passive transport of cations into and anions out of the cell
Electrochemical gradient
two combines forces that drive the diffusion of ions across a membrane
a chemical force (the ion’s concnetration gradient)
an electrical force (the effect of the membrane potential on the ion’s movement)
an ion diffuses down its electrochemical gradient
Electrogenic pumps
a transport protein that generates voltage across a membrane, storing energy that can be used for cellular work
the main electrogenic pump differs between plants and animals
animals use sodium-potassium pumps
Plants, fungi, and bacteria use proton pumps, which actively transports hydrogen ions out of the cell
Cotransporter
couples the movement of H+ back down its concentration gradient to the active transport of sucrose into the cell
this is how plants load sucrose into their veins for transport around the plant body
Receptor-mediated endocytosis
vesicle formation is triggered by solute binding to receptors
receptor proteins bound to specific solutes from the extracellular fluid are clustered in coated pits that form coated vesicles
emptied receptors are recycled to the plasma membrane by the same vesicle
allows the cells to acquire large quantities of a particular substance, even if that substance is not very concentrated in the extracellular fluid
LDL (low density lipoprotein)
delivers lipid molecules to cells from the liver
involved in atherosclerosis, a process in which it is oxidized within the walls of arteries forming plaques
HDL (high density lipoprotein)
unlike the larger lipoprotein particles, which deliver fat molecules to cells, HDL particles remove fat molecules from cells & blood stream and deliver them to the liver
Chylomicrons
transport lipids absorbed from the intestine to adipose, cardiac, and skeletal muscle tissue
triglyceride components are hydrolyzed by the activity of the lipoprotein lipase, allowing the release free fatty acids to be absorbed by the tissues
when a large portion of the triglyceride core has been hydrolyzed, chylomicron remnants are formed and are taken up by the liver, thereby transferring dietary fat to the liver
metabolism
the totality of an organism’s chemical reactions
metabolic pathway
a specific molecule is altered in a series of steps to produce a product
each step is catalyzed by a specific enzyme, a macromolecule that speeds up a specific reaction
Anabolic pathways
consumer energy to build complex molecules from simpler ones (“uphill”)
ex. the synthesis of protein from amino acids
Catabolic pathway
release energy by breaking down complex molecules into simpler compounds (“downhill”)
ex. cellular respiration, the breakdown of glucose in the presence of O2
energy
the capacity to cause change, can be used to do work—move matter against opposing forces, such as gravity and friction
kinetic energy
energy associated with motion
thermal energy
the kinetic energy associated with random movement of atoms or molecules
Potential energy
energy that matter possesses because of its location or structure
Chemical energy
potential energy available for release in a chemical reaction
Energy transformations
chemical energy from food is used to perform the work of climbing up to a diving platform
the kinetic energy of muscle movements is transformed into potential energy as the diver climbs higher above the water
the potential energy is then transformed to kinetic energy as the diver falls back down to the water
The first law of thermodynamics
the energy of the universe is constant
this means that energy can be transferred and transformed, but it cannot be created or destroyed
the first law is also called the principle of conservation of energy
The second law of thermodynamics
during every energy transfer or transformation, some energy is converted to thermal energy and lost as heat, becoming unavailable to do work
every energy transfer or transformation increases the entropy of the universe
Entropy
a measure of molecular disorder, or randomness
may decrease in a particular system, such as an organism, as long as the total entropy of the system and surroundings increases
Spontaneous processes
occur without energy input; they can happen quickly or slowly
increase the entropy of the universe
spontaneous means that a reaction is energetically favorable, not that it will occur rapidly
Nonspontaneous processes
decrease entropy; they require an input of energy
Biological order
cells create ordered structures from less organized starting materials
the increase in order within living systems is balanced by the catabolic breakdown of organized forms of matter, releasing heat and small molecules
Free energy
the portion of a system’s energy that can do work when temperature and pressure are uniform throughout the system as in a living cell
change in free energy during a reaction is related to temperature and changed in enthalpy and entropy
ΔG
can be used to determine whether a process is spontaneous or not
negative for all spontaneous processes
spontaneous decreases free energy—can be harnessed by the cell to perform work
system loses free energy and becomes more stable
positive for nonspontaneous processes
nonspontaneous increases free energy
represents the different between free energy of the final state and free energy of the initial state
Equilibrium
the point at which forward and reverse reactions occur at the same rate, describes a state of maximum stability
systems never spontaneously move away from this
process is spontaneous and can perform work only when it is moving towards this
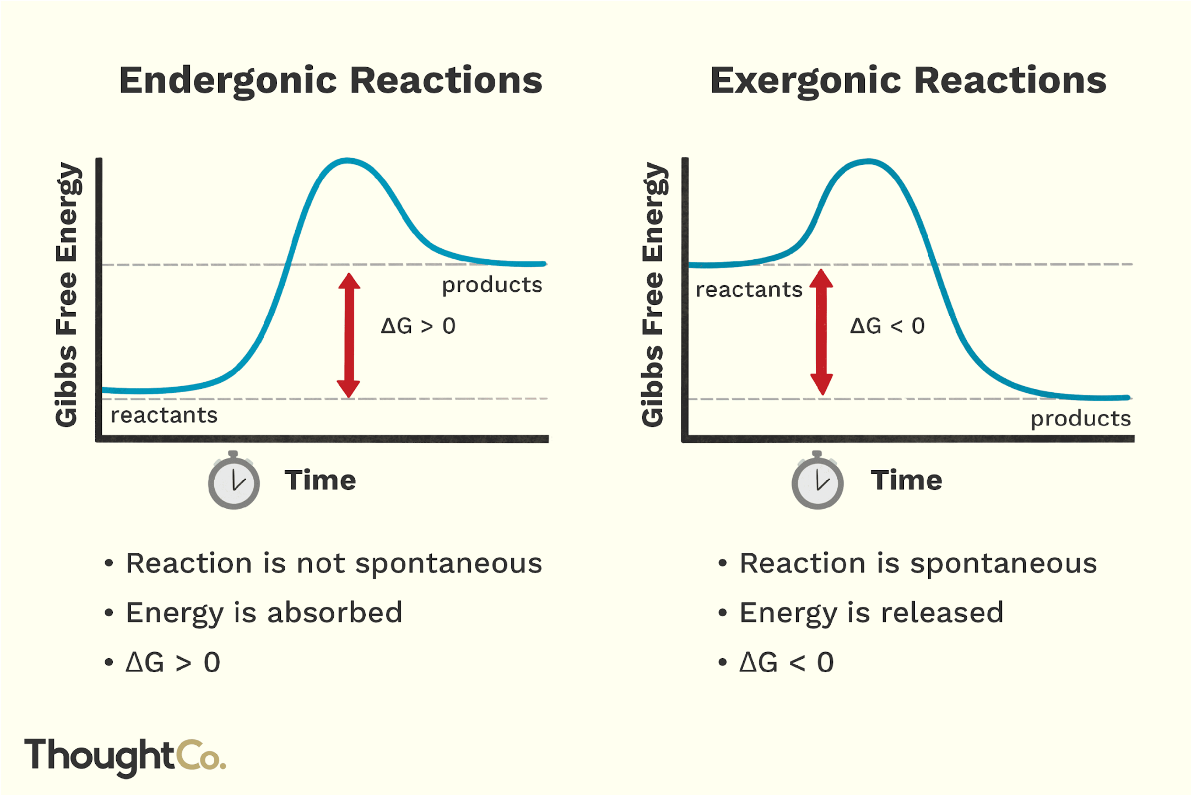
Exergonic reaction (“energy outward”)
proceeds with a net release of free energy to the surroundings
products store less free energy than the reactants
ΔG is negative
because of this, these reactions occur spontaneously
breaking bonds requires energy, not releases—potential energy is released when bonds are formed after the original bonds break
Endergonic reaction (“energy inward”)
absorbs free energy from the surroundings
products store more free energy than the reactants
ΔG is positive
reactions are nonspontaneous
ex. photosynthesis—this reaction is powered by converting light energy to chemical energy
Closed systems
reactions, such as an isolated hydroelectric system, eventually reach equilibrium and can then do no work
Open systems (living things)
chemical reactions of metabolism are reversible, but never reach equilibrium in a living cell
more apt analogy.
a catabolic pathway in a cell releases free energy in a series of reactions
ex. cellular respiration, reactions are “pulled” in one direction because the products of each reaction are the reactants in the next step
steady inflow of glucose and release of waste products ensures that equilibrium is never reached
Transport work
pumping substances across membranes against the direction of spontaneous movement
nearly always powered by ATP hydrolysis
Mechanical work
beating cilia or contracting muscle cells
nearly always powered by ATP hydrolysis
Chemical work
pushing endergonic reactions
ATP powers cellular work
cells manage energy resources to do work through energy coupling, the use of an exergonic process to drive an endergonic one
most energy coupling in cells is mediated by ATP
ATP (adenosine triphosphate)
composed of ribose (a sugar), adenine (a nitrogenous base), and three phosphate groups
in addition to energy coupling, ATP functions as one of the nucleoside triphosphates used to make RNA
ATP hydrolysis
energy is released from ATP when the terminal phosphate bond is broken by hydrolysis, the addition of a water molecule
the energy does not come directly from the phosphate bonds, but from the chemical change to a state of lower free energy in the products
causes a change in protein shape and binding ability
Phosphorylation
transfer of a phosphate group form ATP to another molecule, is typically used to power endergonic reactions
Phosphorylated intermediate
recipient molecule of phosphorylation
is more reactive (less stable, with more free energy) than the original molecule
ATP regeneration
ATP is regenerated by addition of a phosphate group to adenosine diphosphate (ADP)
free energy needed to phosphorylate ADP comes from exergonic breakdown reactions (catabolism)
the shuttling of inorganic phosphate and energy is called the ATP cycle; it couples energy-yielding processes to energy-consuming ones
Catalyst
chemical agent that speeds up a reaction without being consumed by the reaction
Enzyme
a macromolecule (typically protein) that acts as a catalyst to speed up a specific reaction
Activation energy (EA)
the initial energy needed to break the bonds of the reactants
heat in the form of thermal energy absorbed from the surroundings often supplies this
molecules become unstable when enough energy is absorbed to break bonds; this is the transition state
as atoms settle into new, more stable bonds, energy is released to the surroundings
in an exergonic reaction, the formation of new bonds releases more energy than was invested in breaking the old bonds
Activation energy barrier
provides a barrier that determines the rate of spontaneous reactions
for some reactions, EA is low enough that thermal energy a room temperature is sufficient enough to overcome the activation barrier
most reactions have high EA, and need additional energy (usually heat) to reach the transition state
Free energy diagrams
How enzymes speed up reactions
adding heat not useful because it denatures proteins
heat speeds up all reactions—not just ones needed
instead organisms carry out catalysis
Catalysis
the process by which a catalyst selectively speeds up a reaction without itself being consumed
enzyme catalyzes a reaction by lowering the EA barrier enough for the reaction to occur at moderate temperatures
an enzymes cannot change ΔG; it only speeds up a reaction that would eventually occur anyways
Substrate
the reactant that an enzyme acts on
Enzyme-substrate complex
forms from the enzyme binding to its substrate
each enzyme catalyzes a specific reaction and can recognize its specific substrate among even closely related compounds
Active site
the region on the enzyme, often a pocket or groove, that binds to the substrate
when the substrate enters this, the enzyme changes shape slightly, tightening around the substrate like a handshake
induced fit
results from interactions between chemical groups on the substrate and the active site
Catalysis in the enzyme’s active site
substrate is typically held in the enzyme’s active site by weak bonds, such as hydrogen bonds
rate of enzyme-catalyzed reaction can be sped up by increasing substrate concentration
Saturated enzyme
when all enzyme molecules have their active sited engaged
if the enzyme is saturated, the reaction rate can only be sped up by adding more enzyme
Effects of temperature on enzymes
each enzyme has an optimal temperature at which it catalyzes its reaction at the maximum possible rate
up to this point, the reaction rate increases with increasing temperature; beyond this point the rate of reaction begins to drop
Effects of pH on enzymes
each enzyme has an optimal pH that is dependent on the environment in which it is typically active
ex. the optimal pH for pepsin—a human stomach enzyme—is 2, whereas the optimal pH for trypsin—an intestinal enzyme—is 8
Cofactors
are nonprotein helpers that bind to the enzyme permanently, or reversibly with the substrate
inorganic cofactors include metal atoms such as zinc, iron, and copper ionic form
Coenzymes
organic cofactors
Competitive inhibition
competitive inhibitors closely resemble the substrate and can bind to the enzyme’s active site
enzyme productivity is reduced because the inhibitor blocks the substrate from entering the active site
increasing substrate concentration can overcome this type of inhibition
Noncompetitive inhibition
noncompetitive inhibitors bind to another part of the enzyme, away from the active site
binding of the inhibitor causes the enzyme to change shape,
making the active site less effective at catalyzing the reaction
noncompetitive inhibitors bind to the enzyme regardless of whether the substrate is attached yet; the inhibitor can EITHER bind to the enzyme or the enzyme-substrate complex
Regulation of enzymes helps control metabolism
chemical chaos would result if a cell’s metabolic pathways were operating simultaneously
cells can regulate metabolic pathways by switching on or off the genes that encode specific enzymes, or by regulating the activity of existing enzymes
Allosteric regulation of enzymes
occurs when a regulatory molecule binds to a protein at one site and affects the protein’s function at another site
most of these enzymes are made from polypeptide subunits, each with its own active site
the complex oscillates between two shapes, one catalytically active and the other inactive
may either inhibit or stimulate enzyme’s reaction rate
Binding of an activator stabilizes the shape that has functional active sites, while binding of an inhibitor stabilizes the inactive form of the enzyme
Cooperativity
substrate binding to one active site triggers a shape change in the enzyme that stabilizes the active form for all other sites
this mechanism amplifies the response by priming the enzyme to act on additional substrate molecules more readily
Feedback inhibition
the end product of a metabolic pathway shuts down the pathway
prevents a cell from wasting chemical resources by synthesizing more product than is needed
Localization of enzymes
compartmentalization of the cell helps to bring order to metabolic pathways
in some cases, the enzymes for several steps in a metabolic pathway form a multienzyme complex
some enzymes have fixed locations and act as structural components of particular membranes
Catabolic pathways yield energy by oxidizing organic fuels
energy enters ecosystems as light and exits as heat
the chemical elements essential to life are recycled
catabolic pathways release stored energy by breaking down complex molecules
electron transfer from food molecules to other molecules plays a major role in these pathways
Fermentation
a partial degradation of sugars that occurs without oxygen
Aerobic respiration
consumes organic molecules and oxygen and yields ATP
Anaerobic respiration
similar to aerobic respiration but consumes compounds other than oxygen
Cellular respiration equation
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O + Energy (ATP + heat)
Cellular respiration
includes both aerobic and anaerobic respiration but is often used to refer to aerobic respiration
although carbohydrates, fats, and proteins are all consumed as fuel, it is helpful to trace cellular respiration with the sugar glucose
Why? Because glucose is the only molecule that fuel the first step called glycolysis
catabolic pathways do not directly power work in the cell; they are linked to work by ATP
Cells must constantly regenerate their supply of ATP from ADP and phosphate
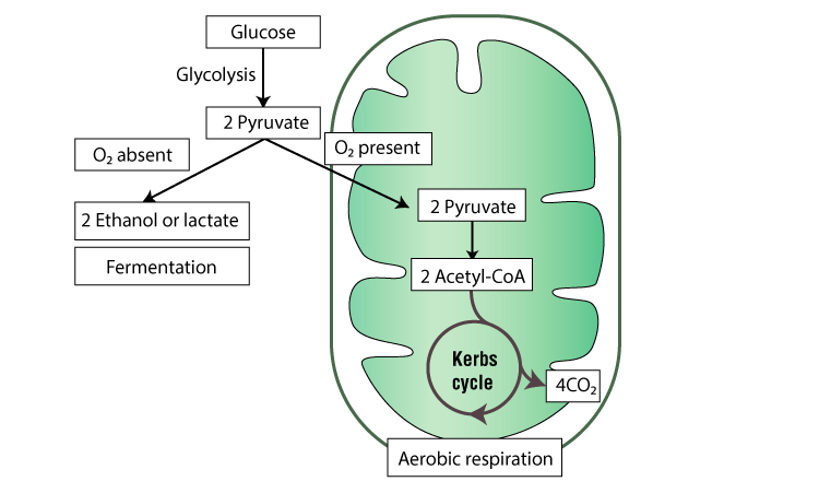
Stages of Cellular Respiration: Preview
Harvesting energy from glucose by cellular respiration has three stages
Glycolysis breaks down glucose into two molecules of pyruvate
Pyruvate oxidation and the citric acid cycle complete the breakdown of glucose to CO2
During oxidative phosphorylation the electron transfer chain and chemiosmosis facilitate the synthesis of most of the cell’s ATP
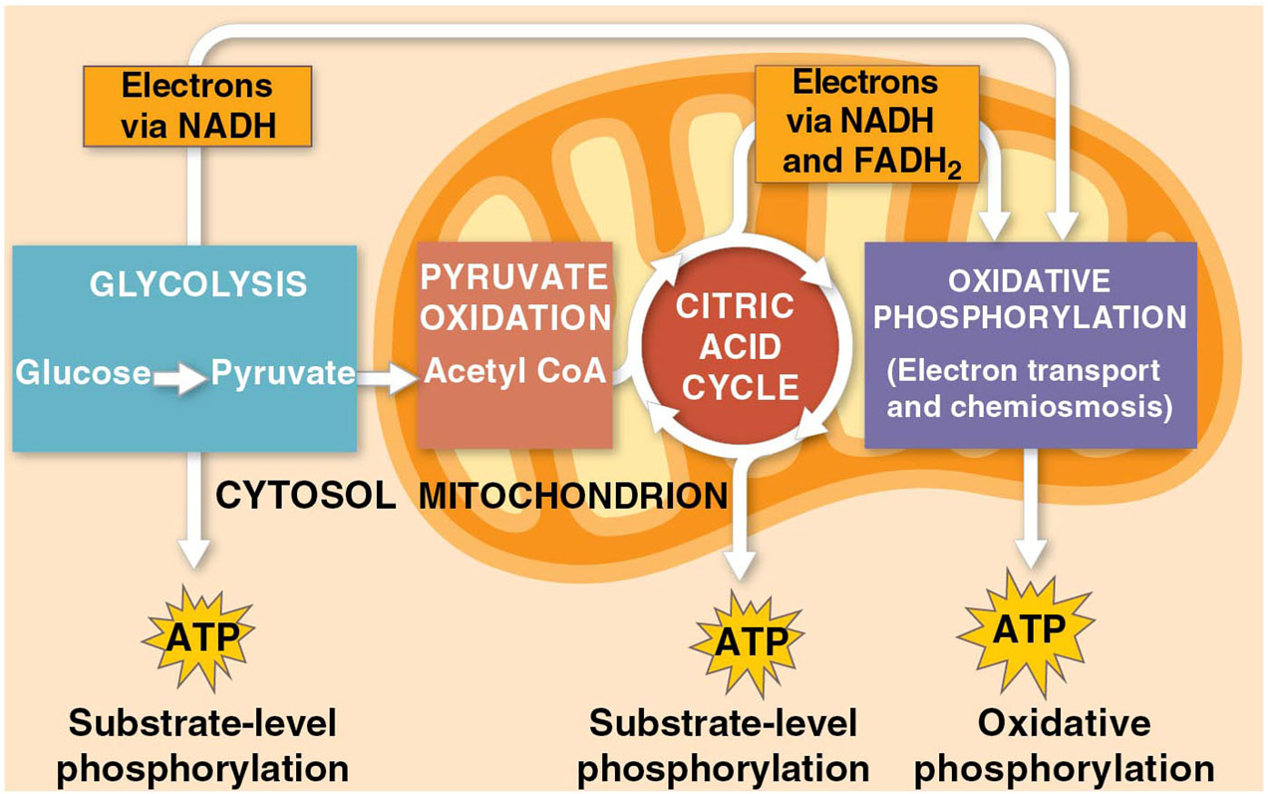
Substrate level phosphorylation
forms some ATP in glycolysis and the citric acid cycle
occurs when an enzyme transfers a phosphate group directly from a substrate to ADP
Oxidative phosphorylation
process that generates almost 90% of ATP because it is powered by redox reactions
Oxidation of organic fuel molecules during cellular respiration
during cellular respiration, fuel molecules (such as glucose) are oxidized (broken down) and O2 is reduced (made more complex/into compound)
organic molecules with an abundance of hydrogen are excellent sources of high-energy electrons
cellular respiration is a redox process; energy is released as hydrogen and electrons are transferred to O atoms
the oxidation of glucose transfers electrons from a higher energy state (in glucose) to a lower energy state with O atoms
this releases energy that is used to synthesize ATP
NAD+
a coenzyme that functions as an electron carrier
as an electron acceptor, functions as an oxidizing agent during cellular respiration
each NADH (reduced form) represents stored energy that is tapped to synthesize ATP
each electron travels with a proton—thus, as a hydrogen atom
Stepwise energy harvest via NAD+ and the electron transport chain
enzymes called dehydrogenases remove a pair of hydrogen atoms (2 electrons and 2 protons) from the substrate
the 2 electrons and 1 proton is transferred to NAD+ forming NADH
the other proton is released as a hydrogen ion (H+) into the surrounding solution
Electron transport chain
If NADH transferred electrons directly to oxygen, energy would be released in one explosive reaction
instead, cellular respiration uses an energy transport chain to break the fall of electrons to O2 into several energy-releasing steps
an electron transport chain consists of a series of molecules built into the inner membrane of the mitochondria (or plasma membrane of prokaryotes)
NADH passes electrons to the electron transport chain where they are transferred in a series of redox reactions, each releasing a small amount of energy
O2, the final electron acceptor, captures the electrons and the hydrogen nuclei (H+), forming H2O
The energy yielded is used to regenerate ATP