Topic 7 Enzyme mechanism and inhibition
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
How is enzyme activity modulated by temperature and PH
PH
If PH decreased activity will decrease
Changing PH may change protonation state of our ionizable groups; maybe we can’t accept or donate a proton somewhere anymore when working in acid-base catalysis.
Temperature
Increasing temperature denatures and solidifies protein
Low temp= less thermal fluctuation
What are the different types of reversible enzyme inhibition by specific molecules examples?
Competitive inhibitor
Uncompetitive inhibitor
Noncompetitive inhibitor
What does inhibition mean in biochem?
inhibition refers to the process by which the activity of an enzyme is reduced or stopped-usually by a molecule known as an inhibitor
This inhibitor can bind to the enzyme and interfere with its ability to catalyze a reaction
Explain “Enzyme inhibition- Competitive inhibitors”
Have structures similar to the substrate
Bind to the active site, reducing the amount of available enzyme for the substrate
(If an inhibitor is bound to the active site of an enzyme, a substrate can’t also bind)
Useful in drug design because many drugs are designed as competitive inhibitors because they can selectively block enzymes involved in disease pathways
No effect on V-max, so full enzyme activity can be restored if needed
Explain “Enzyme inhibition- Competitive inhibitors” graph compared to normal rxn
No effect on V-max, so full enzyme activity can be restored if needed
More substrate needed to reach V-max/2
Adding more substrate [S] will outcompete inhibitor [I]
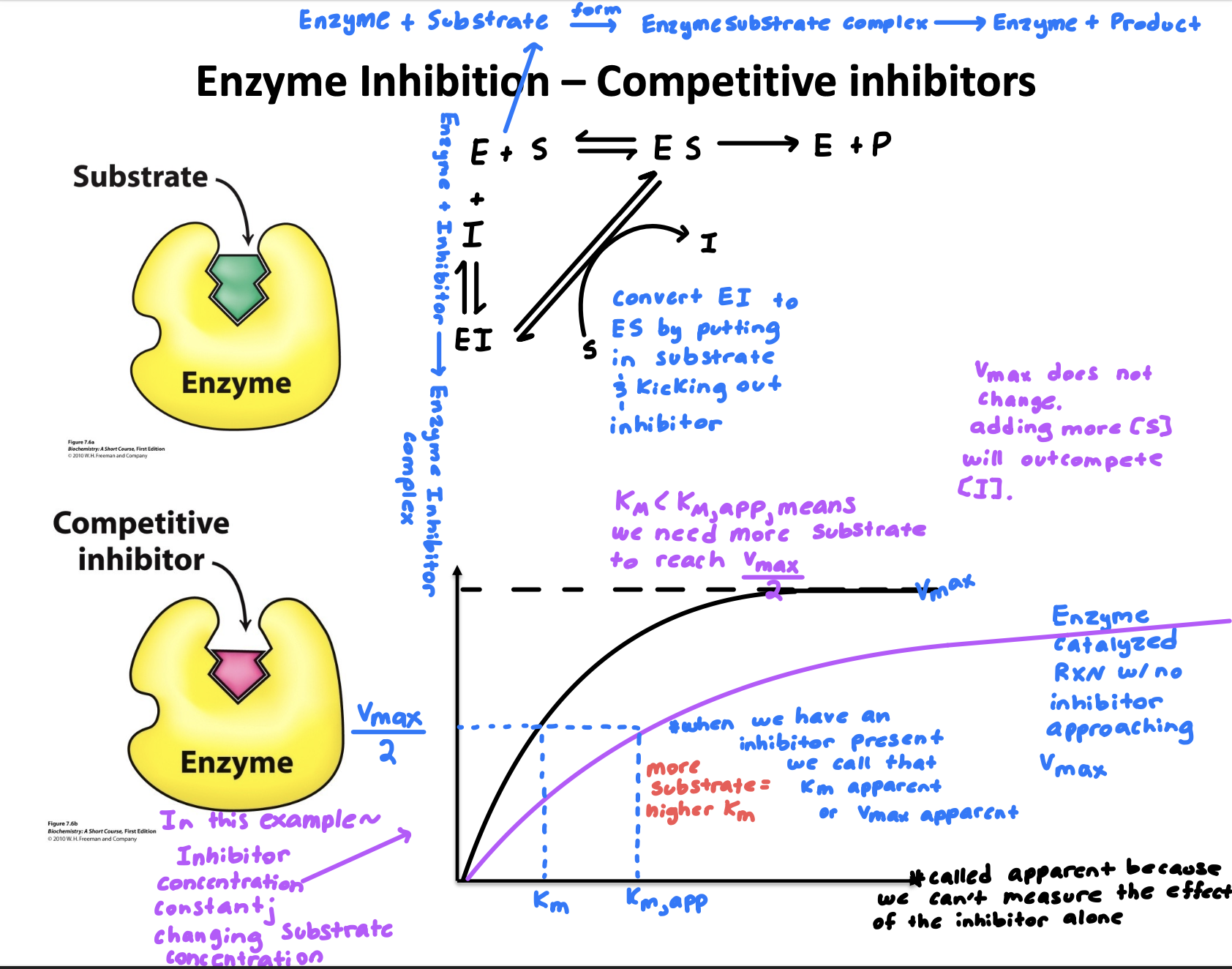
What does apparent mean ?
When an inhibitor is present we call that Km apparent or V-max apparent
Define Km?
Substrate concentration at ½ Vmax
Low Km= high substrate affinity
High Km= Low substrate affinity
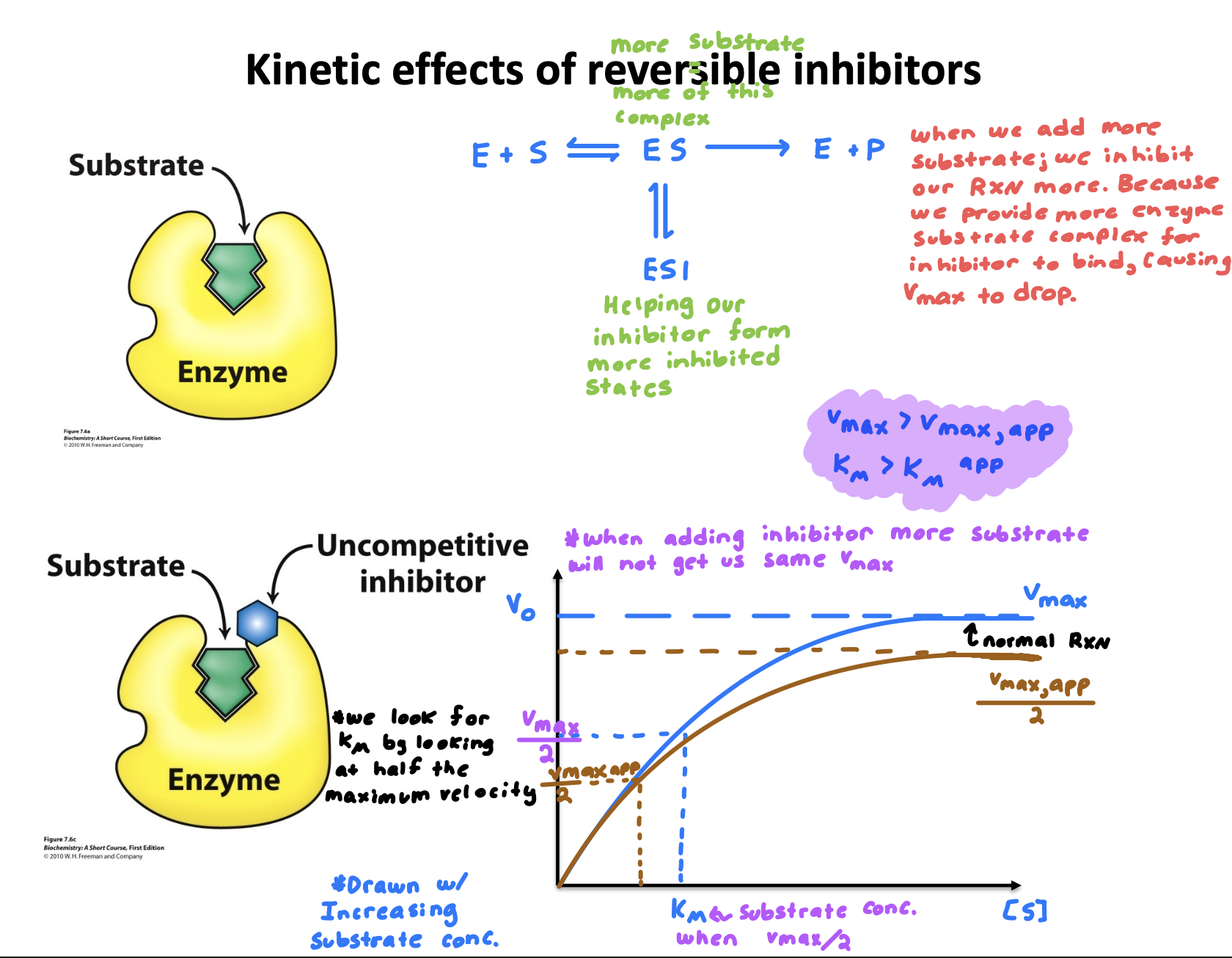
Explain “Enzyme inhibition- Un-competitive inhibitors”
Binds only to enzyme-substrate complex; not enzyme alone.
cannot overcome by increase in [S] because if we add more substrate we form more ES which I is able to bind to
Explain “Enzyme inhibition- Un-Competitive inhibitors” graph compared to normal rxn
When we add more substrate; we inhibit our rxn more, because we provide more enzyme substrate complex for the inhibitor to bind to, causing V-max to drop.
Km apparent is less with inhibitor than normal reaction due to drop in V-max
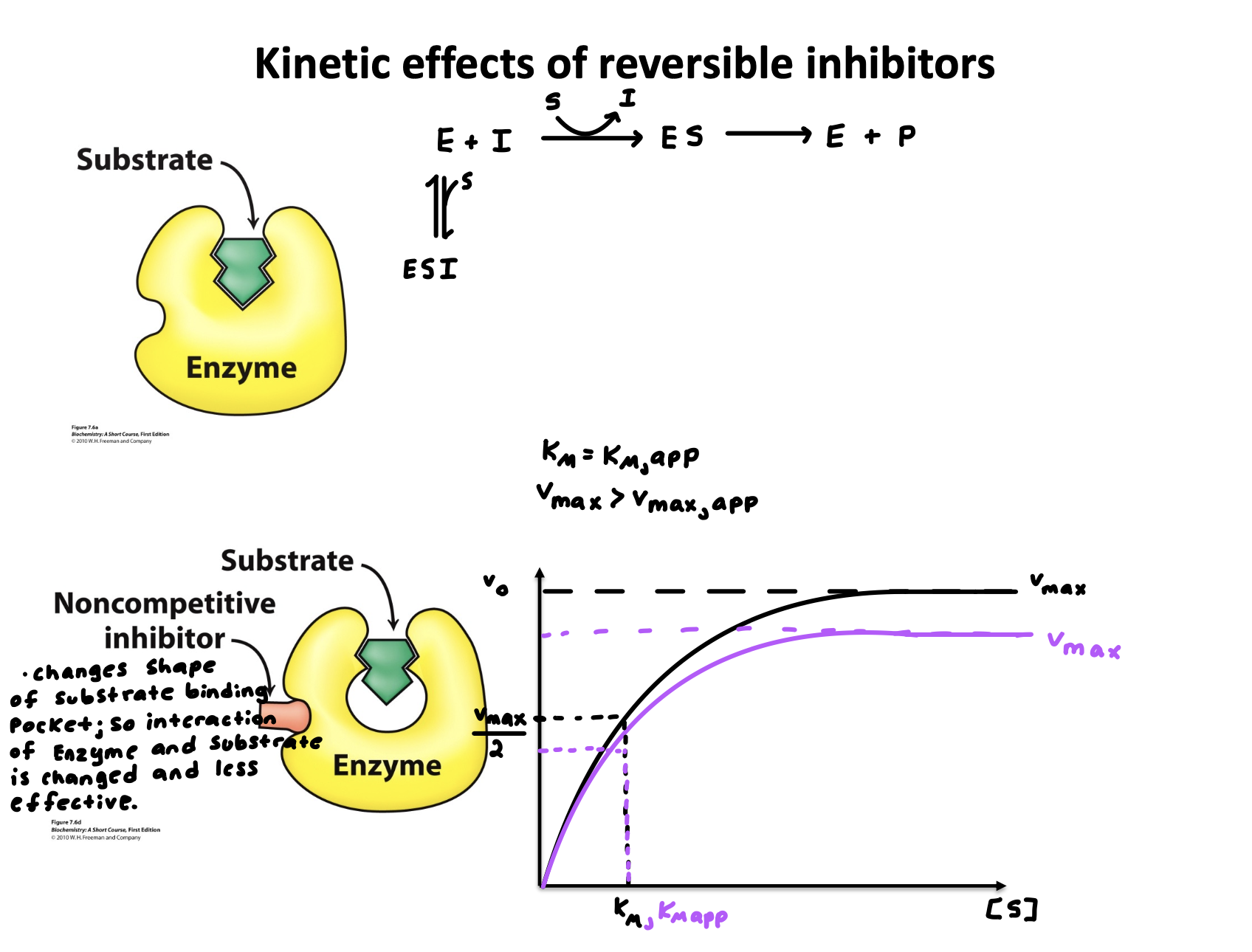
Explain “Enzyme inhibition- Non-Competitive inhibitors”
Changes the shape of substrate binding pocket; so interaction of Enzyme and substrate is changed and less effective
binds to an allosteric site (not active site)
Explain “Enzyme inhibition- Non-Competitive inhibitors” graph compared to normal reaction
Km= Km,app (Km stays the same because substrate binding affinity doesn’t change)
V-max > V-max,app (V-max goes down because less enzyme is active)
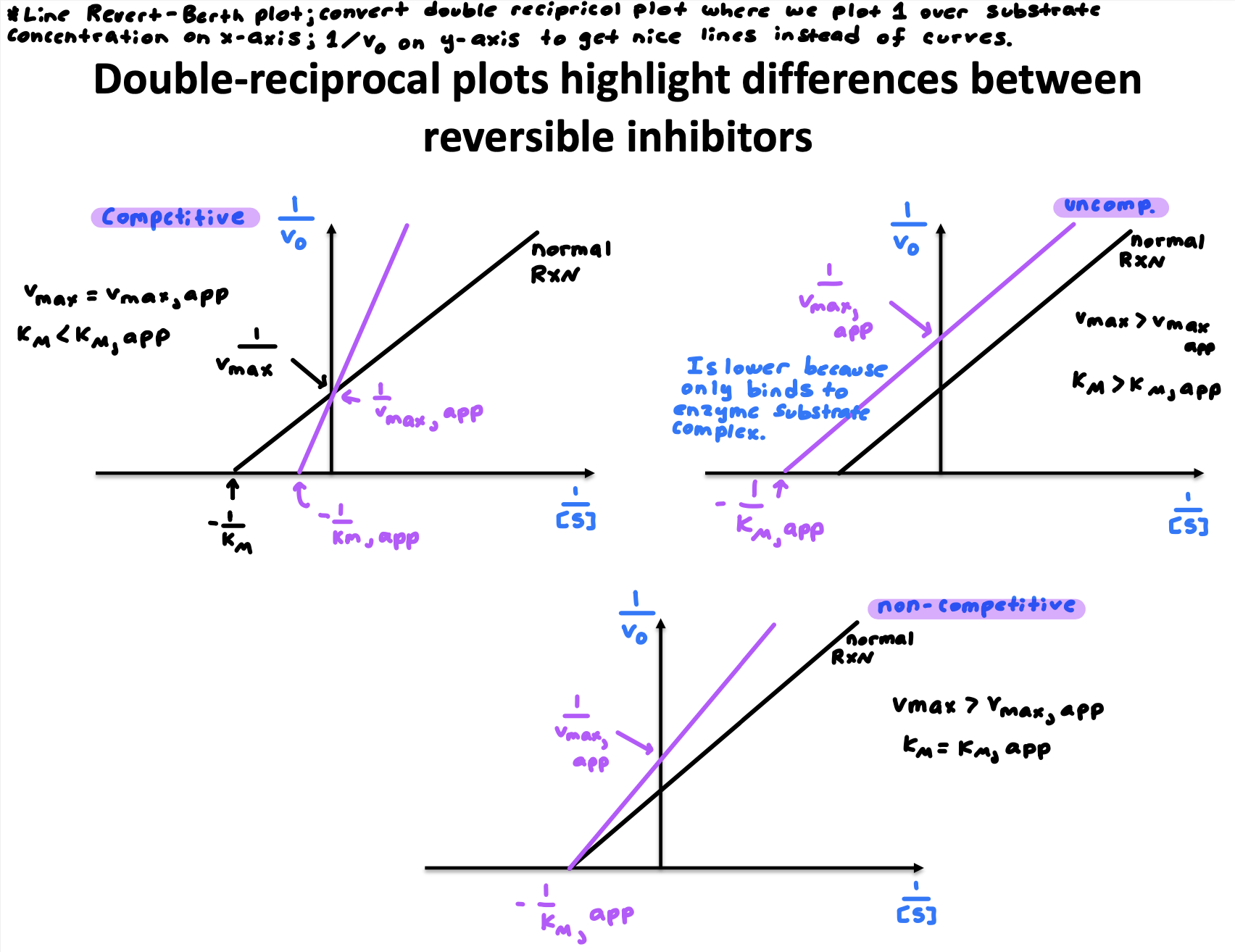
How would the double-reciprocal plots between reversible inhibitors look?
What is a protease? why is it necessary for protein turn over? Where does its substrate specificity come from?
protease: An enzyme that catalyzes the cleavage of peptide bonds using a hydrolysis reaction. (Example- Chymotrypsin)
Protease is necessary for protein turnover because they break down proteins into smaller peptides or amino acids enabling the cell to recycle amino acids, remove misfolded proteins, and regulate biological processes’s.
Its substrate specificity comes from the structure of its active site which binds specific amino acids, in this case it likes to break peptide bonds on hydrophobic AA residues.
What is chymotrypsin?
Chymotrypsin is a digestive enzyme that is made in the pancreas
Chymotrypsin is a protease, that cleaves peptide bonds after large hydrophobic amino acids
(it breaks down proteins into smaller peptides for easier digestion)
Serine-195, Histidine-57, and Aspartate-102 are residues on the chymotrypsin protein that form this catalytic triad that cleaves the peptide bond.
What is required for Chymotrypsin activity?
Catalytic triad residues (essential for catalysis)
Serine 195 (nucleophile) stouts reaction
Histidine 57 (acts as a base) abstracts a H+ from Serine 195
Aspartate 102 (stabilizes Histidine) fixing geometry
Presence of substrate
the enzyme specifically cleaves after bulky, hydrophobic amino acids like Phenylalanine, tyrosine, and tryptophan
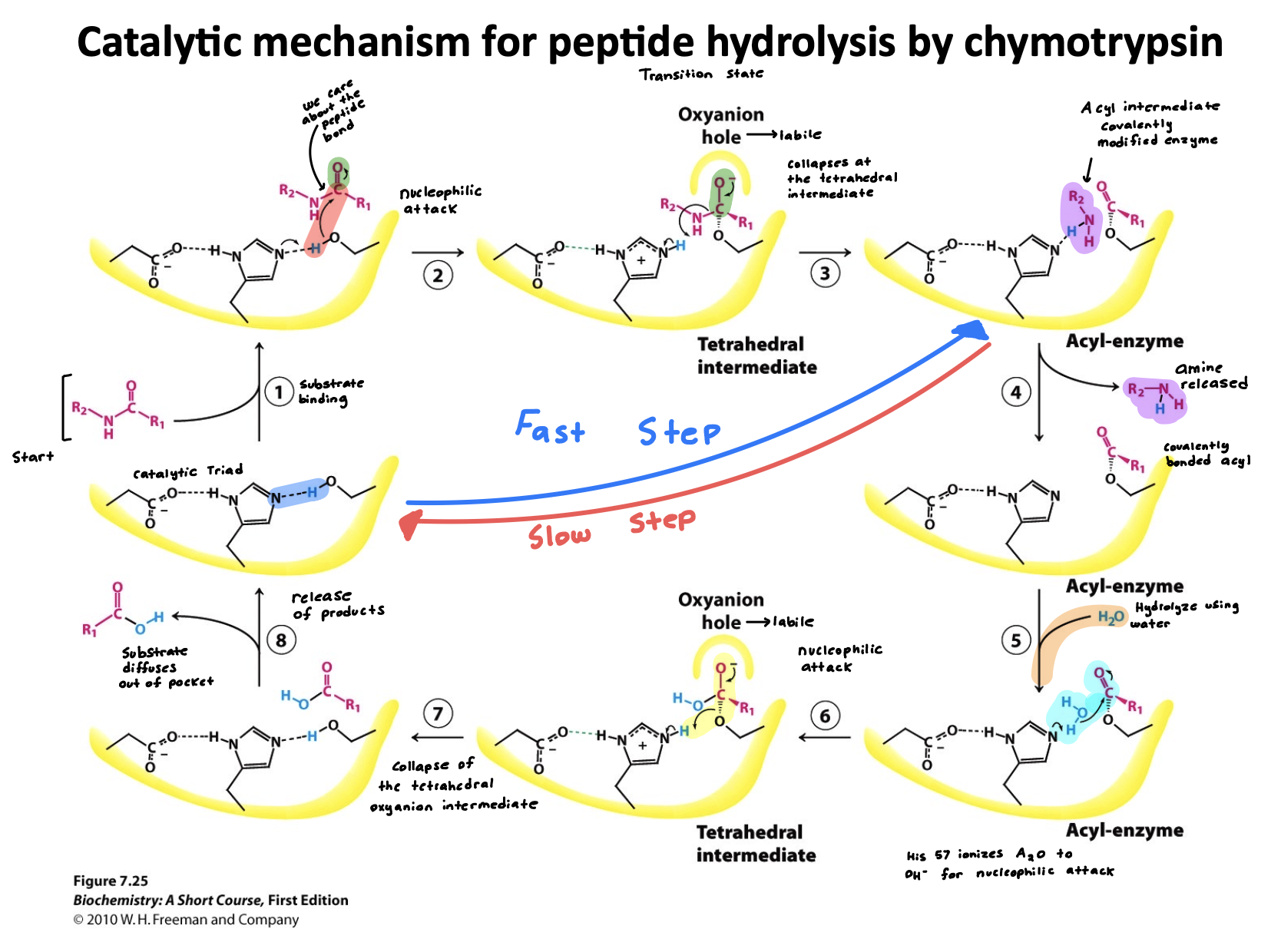
Explain Chymotrypsin mechanism.
Two step reaction
Step 1: Acylation (fast)
Acylation is the addition of R-CO- ; in this case its to Serine-195 where it becomes covalently bonded.
Aspartate-102 stabilizes the positive charge on Histidine by H-bonding to it(enhancing its ability to de-protonate)
Histidine-57 acts as the base accepting protons from Serine-195 to activate it as a nucleophile
The oxyanion Hole is found within both these phases, it ultimately forms a stabilizing pocket that stabilizes the negative charge on oxygen during the intermediate step
Step 2: De-acylation (slow)
In De-acylation R-CO- ; becomes removed from Serine-195 restoring the enzyme ultimately.
Aspartate-102 stabilizes the positive charge on Histidine by H-bonding to it (enhancing its ability to de-protonate)
Histidine-57 acts as the base again for water activating it as the nucleophile to attack the acyl-enzyme intermediate.
The oxyanion Hole is found within both these phases, it ultimately forms a stabilizing pocket that stabilizes the negative charge on oxygen during the intermediate step
Summary:
whole point of this regeneration in Chymotrypsin is to allow the enzyme to be reused repeatedly-catalyzing many reactions without being consumed. Focusing on efficiency.
What is a chromogenic substrate? why is this important?
A chromogenic substrate makes chymotrypsin activity visible.
The chymotrypsin cleaves the peptide bond next to an amino acid it prefers and released a product, which is yellow.
This is important because the colored product allows you to quantify the enzyme activity by measuring the absorbance with Uv-vis.
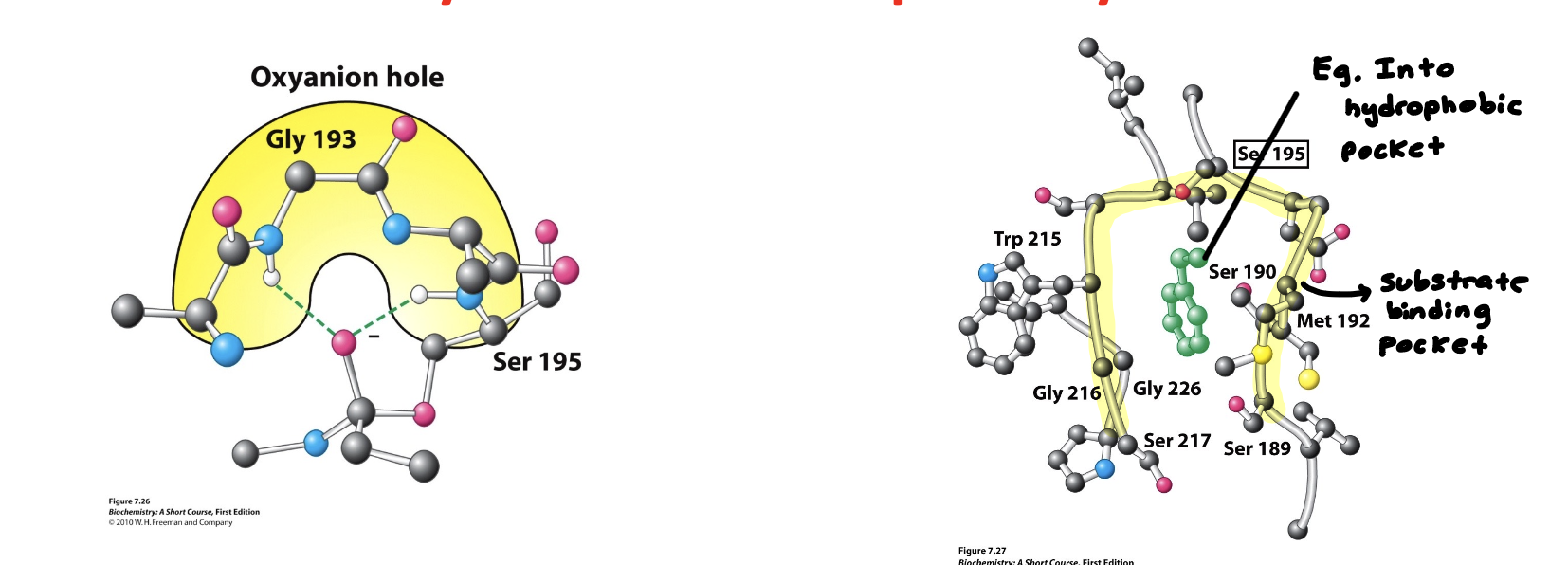
Explain important structural features of Chymotrypsin “The Oxyanion hole” and “Specificity Pocket”
The Oxyanion hole
Stabilizes the transition state by stabilizing/bonding with the negative charge of the other wise labile (High unstable and short lived) transition state
Specificity Pocket or Substrate binding pocket
Important for specificity
Chymotrypsin binds to really large hydrophobic groups
Substrate binding pocket is lined with hydrophobic residues that promote the binding of hydrophobic substrates
(learning objective) Describe the catalytic cycle of chymotrypsin?
the whole outcome of this cycle is peptide bond hydrolysis (large proteins are broken into smaller peptides)
The reaction is broken into two phases a fast and slow one
Step 1: acetylation
Serine attacks and peptide bond is cleaved
First product is released
Acyl-enzyme intermediate is formed
Step 2: de-acetylation
Water attacks acyl-enzyme
Second product is released
Enzyme is re-generated
(learning objective) Why are there two kinetic phases?
There are two kinetic phases because the peptide bond cleavage and acyl intermediate is fast. Hydrolysis of acyl intermediate is what takes longer.
(learning objective) What are they key structural features that allow chymotrypsin to have substrate specificity? How does it stabilize the transition state?
Key structural feature for specificity is the hydrophobic pocket (substrate binding pocket) that binds to hydrophobic side chains line Phe, Tyr, and Trp
Oxyanion hole stabilizes the negative charge on the tetrahedral intermediate
(learning objective) describe the role of each amino acid in the catalytic triad?
Serine 195- acts at the nucleophile
Histidine 57- acts as the base
Aspartate 102- stabilizes the charge on histidine