ex 3 Dissolution Testing of Amoxicillin Capsules and Aspirin Tablets
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
Dissolution test
FDA requirement in testing solid dosage forms, such tablets and capsules
Dissolution test
Measures the rate of release of active pharmaceutical ingredients into solution from the final pharmaceutical product.
Rotating basket assembly (Apparatus I)
apparatus in dissolution test for hard and soft gelatin cap
Rotating basket assembly (Apparatus I)
process by which a chemical or a drug becomes dissolved in a solvent.
Dissolution rate
the amount of active ingredient in a solid-dosage form dissolved in unit time under standardized conditions of liquid/solid interface, temperature and media composition.
1. Aqueous medium
2. Temperature of the medium
3. Agitation rate
Factors affecting the dissolution rate
• Quality Control
1. Batch homogeneity
2. Batch to batch conformity
3. Stability
4. Therapeutically optimal dosage forms
• Research & Development
1. 2. 3. Drug release behavior of pre-formulations
In vitro simulation of the GI passage
IVIVC (in vitro-in vivo correlation)
Importance of Dissolution Test
1. Proper alignment of the dissolution apparatus
Proper conditions during dissolution test
a) Temperature (37°C)
b) Agitation speed
c) Sampling (sampling zone, timing, filtration, dilution)
d) Vibration (plane area, stable, drive chain & belts should be free of
tension & dirt, external vibration)
e) Dissolution medium (pH, surface tension & viscosity of dissolution
medium
Proper validation of analytical method
Factors affecting the Test reliability
1. speed or rpm of the rotation of the basket
kind and volume of dissolution media
3. time of run
wavelength of the spectrophotometer in absorbance readings
Factors affecting the Test reliability
Aside from temperature, there are other parameters that must becontrolled;
Vessel
Motor
Mettalic shaft
Cylindrical basket
Vessel Evaporation Cover
Components of Basket Apparatus
Vessel
(Basket Apparatus)
• Made up of glass or other inert transparent material
• Cylindrical with hemispherical bottom
• 1L, 2L or 4L nominal capacity
• Partially immersed in water bath
(37 + 0.5 o C)
Metallic shaft
(Basket Apparatus)
• Positioned so that its axis in NMT 2mm from the vertical axis of the vessel
• Rotates smoothly without significant wobble
• Made of stainless steel 316
Cylindrical basket
(Basket Apparatus)
• 40 mesh
• Made of stainless steel 316 (gold plating is allowed)
• Distance between inside bottom of vessel and bottom of basket is maintained at 25 + 2 mm
Vessel Evaporation Cover
(Basket Apparatus)
• fitted cover to retard evaporation
Capsule sinker
Components of Paddle Apparatus
Paddle Apparatus
Similar to Basket Appratus, except that a paddle formed from a blade and shaft is the stirring element
Capsule sinker
(Paddle Apparatus)
• A small, loose piece of non-reactive material, such as not more than a few turns of wire helix, may be attached to dosage units that would otherwise float".
-
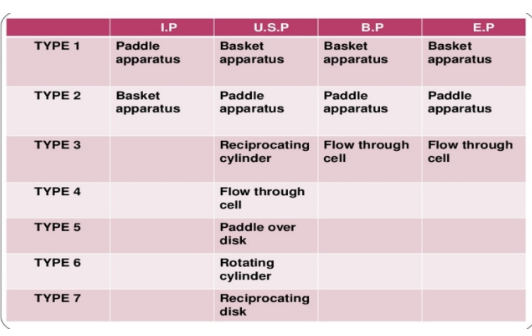
Apparatus 1 and 2
(apparatus) greatly affected by mechanical factors
Apparatus 3
(apparatus) based on the principle of disintegration tester, has hydrodynamic features.
Apparatus 4
(apparatus) forces dissolution medium through cell. Best use for low solubility drugs such as implants, suppositories, and Controlled Release formulations.
Apparatus 5
(apparatus) is also known as sandwich assembly. The dosage form is covered by screen (released side up) and commonly used for transdermal patches.
Apparatus 6
(apparatus) almost the same with apparatus 1 except to replace the basket with a stainless steel cylinder stirring element. It usually placed the dosage form, release side out.
Apparatus 7
(apparatus) is described as flat bottomed cylindrical vessel; medium: 32+0.5 oC
10 ug/ml or 0.01mg/ml
concetration of aspirin reference standard solution
50rpm, 90 minutes
assembly and operation of the dissolutiom apparatus