Limiting Reactants, Reaction Yield, and Solution Concentration in Chemistry
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
67 Terms
Limiting Reactant
The reactant in a chemical reaction that limits the amount of product that can be formed. The reaction will stop when all of the limiting reactant is consumed.
Percent Yield
Actual yield is often less than the theoretical yield. Percent yield is calculated using the formula: actual yield = %yield/100% x Theoretical yield.
Balanced Equation for Ammonia Reaction
4 NH3 (g) + 5 O2 (g) → 4 NO (g) + 6 H2O (l)
Molar Mass of NH3
17 g/mol
Molar Mass of O2
32 g/mol
Moles of NH3 from 2 g
2 g / 17 g/mol = 0.118 mol of NH3
Moles of O2 from 4 g
4 g / 32 g/mol = 0.125 mol of O2
Amount of NO produced
0.118 mol of NH3 produces 0.118 mol of NO.
Amount of H2O produced
0.1 mol of O2 produces 0.15 mol of H2O (6/4 x 0.1 mol).
Excess NH3 remaining
0.018 mol of NH3 remains after the reaction.
Reaction of SiO2 and Carbon
100 grams of SiO2 react with an excess of powdered carbon to form 51.4 grams of SiC.
Limiting Reagent in Water Formation
The reactant that produces the lesser amount of product is the limiting reagent.
Reaction of Silicon Dioxide and Hydrogen Fluoride
SiO2 + 4HF → SiF4 + 2H2O
Molar Mass of SiO2
60 g/mol
Molar Mass of HF
20 g/mol
Moles of SiO2 from 30 g
30 g of SiO2 is 30 g / 60 g/mol = 0.5 mol of SiO2.
Moles of HF from 30 g
30 g of HF is 30 g / 20 g/mol = 1.5 mol of HF.
Grilling Dilemma
At Costco, you can only buy 8 buns, 15 patties, and 44 cheese slices.
Moles of Silicon Tetrafluoride
How many moles of silicon tetrafluoride would be formed from the reaction between 4.5 mol of silicon dioxide and 6.0 mol of hydrogen fluoride?
Hydrogen and Oxygen Reaction
2H2(g) + O2(g) → 2H2O(g)
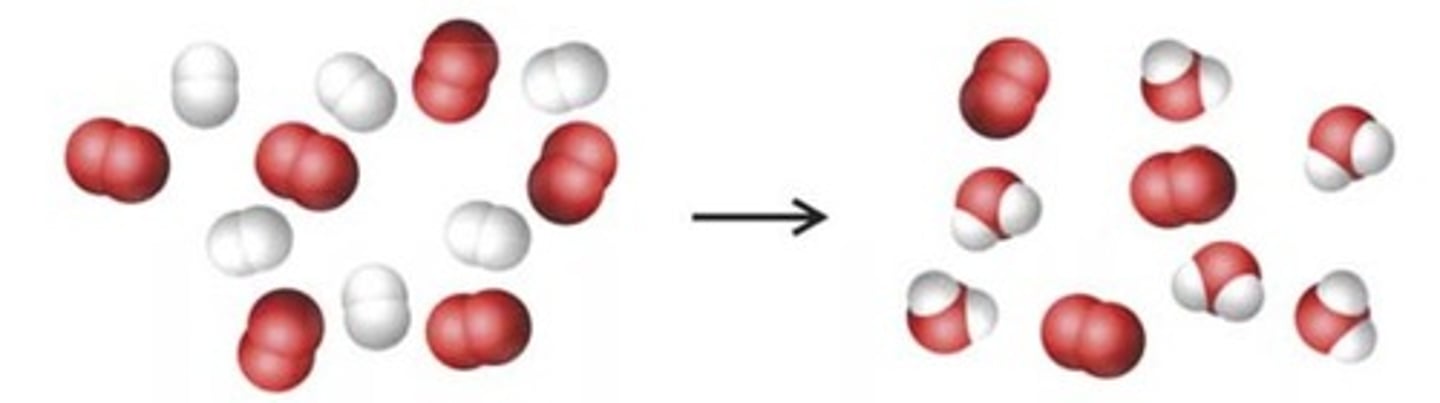
Molar mass of SiC
40.1 g/mol
Theoretical yield of SiC
1.66 mol → 1.66 x 40.1 = 66.7 g
Actual yield of SiC
51.4 g
% yield
% yield = (51.4/66.7) x 100% = 77.1%
Reaction (I)
N2 (g) + 3 H2 (g) → 2 NH3 (g)
Reaction (II)
4 NH3 (g) + 5 O2 (g) → 4 NO (g) + 6 H2O (l)
Moles of SiO2
100/60.1 = 1.66 mol
Left over N2
0.3 moles
Left over NH3
1.4 moles
Moles of NH3 produced from N2
2.0 moles N2 x (2/1) = 4.0 moles NH3
Moles of NH3 produced from H2
5.1 moles H2 x (2/3) = 3.4 moles NH3
Moles of NO produced from NH3
3.4 moles NH3 x (4/4) = 3.4 moles NO
Moles of NO produced from O2
2.5 moles O2 x (4/5) = 2.0 moles NO
Moles of H2O produced from O2
2.5 moles O2 x (6/5) = 3.0 moles H2O
Limiting reactant in Reaction (I)
H2 is the limiting reactant.
Limiting reactant in Reaction (II)
O2 is the limiting reactant.
Q1
What are the amounts of NO and H2O produced?
Q2
What else remains after the reaction?
Solutions
Homogeneous mixtures composed of two or more substances uniformly dispersed at the molecular or atomic level.
Solvent
The substance present in larger quantity that dissolves the solute.
Solute
The substance present in smaller quantity that is dissolved in the solvent.
Solubility
The ability of a substance (solute) to dissolve in a solvent to form a solution, influenced by factors such as temperature, pressure, and the nature of the solvent and solute.
Concentration
Describes the amount of solute dissolved in a given amount of solvent or solution, expressed in various units.
Molarity
The number of moles of solute per liter of solution.
Molality
The number of moles of solute per kilogram of solvent.
Mass percent
The mass of solute divided by the mass of solution, multiplied by 100.
Parts per million (ppm)
A unit of concentration equivalent to 1 milligram of solute per liter of water (mg/l) or 1 milligram of solute per kilogram of soil (mg/kg).
Parts per billion (ppb)
A unit of concentration equivalent to 1 gram of solute per 1 billion grams of solution.
Molar concentration calculation
M = moles of solute / volume of solution in liters.
Example of molarity calculation
For a 1 liter solution with 0.5 moles of solute, M = 0.5 mol / 1 L = 0.5 M.
Example of molarity with glucose
A 100.5 mL solution contains 5.10g glucose (C6H12O6).
Moles of Solute
Moles = Molarity × Volume (L)
Conversion of grams to moles
5.10 g glucose x 1 mole glucose / 180.16 g glucose = 0.0283 mol glucose
Conversion of mL to L
100.5 mL = 0.1005 L
Molarity Calculation Example
1 M = 5.10g (glucose) / (180.16 g glucose × 0.1005 L) = 0.282M
Stock Solution
Concentrated solution (i.e., high solute-to-solvent ratio).
Dilution
Preparation of dilute solution (i.e., low solute-to-solvent ratio) by adding solvent to a given volume of stock solution.
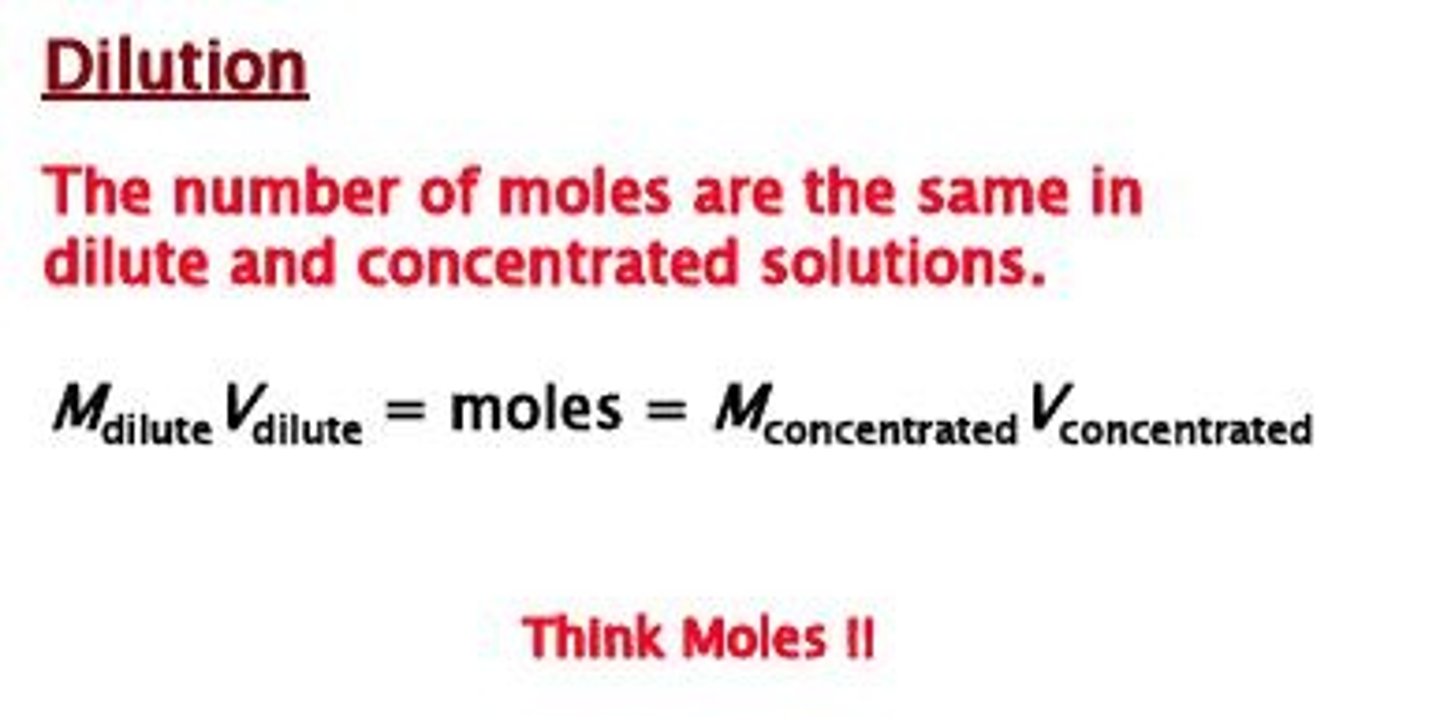
Dilution Process
Dilution refers to the process of reducing the concentration of a solution by adding more solvent.
Electrolyte
A solute that produces ions in solution.
Electrolytic Solution
A solution that conducts electricity.
Charge Carriers
Ions
Non-Electrolytes
Substances in which no ionization occurs. There is no conduction of electrical current.
Examples of Non-Electrolytes
Aqueous solutions of sugar, ethanol, ethylene glycol.
Strong Electrolytes
Nearly 100% dissociated into ions and conduct current efficiently.
Examples of Strong Electrolytes
Solutions of NaCl, HNO3, HCl.
Weak Electrolytes
Only partially dissociate into ions and are slightly conductive.
Examples of Weak Electrolytes
Vinegar (aq. solution of acetic acid); tap water.