history of the atom
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
Democritus 430 BC
Matter is formed of small pieces that could not be cut into smaller parts called atomos (uncuttable)
Dalton 1808
Atoms are invisible particles
atoms of one element are all the same
atoms of different element are different
compounds form by combing atoms
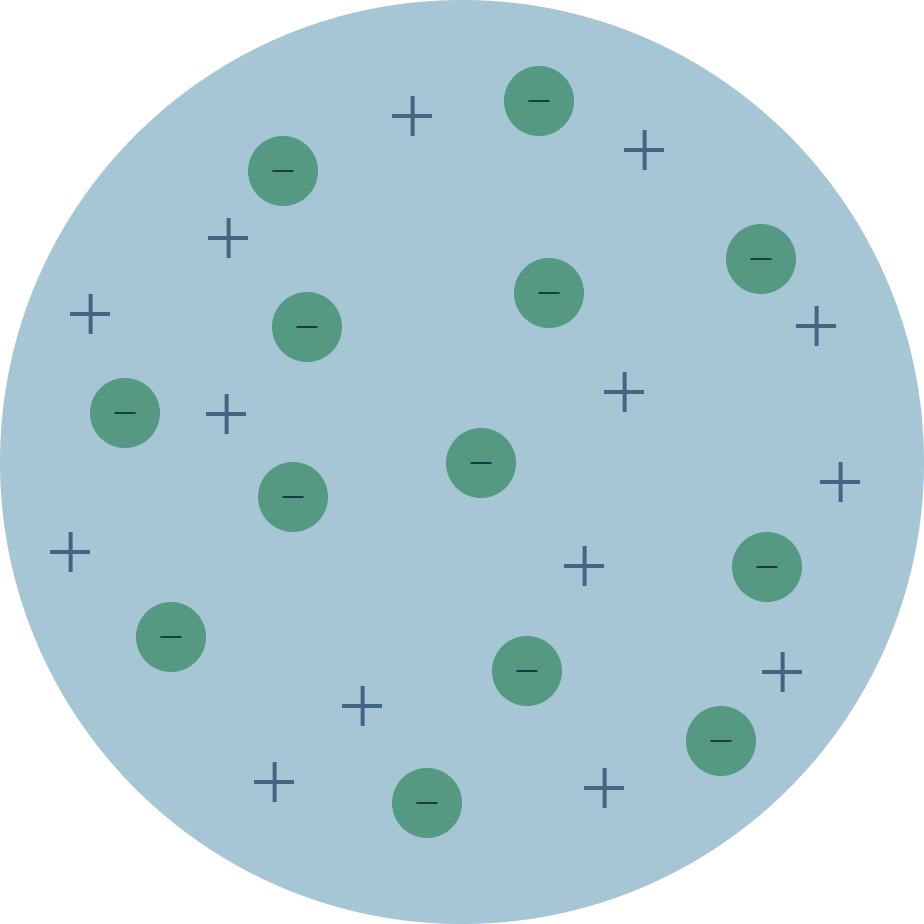
Thomson 1847
Atoms are made mostly out of (+) charged material, like dough in a bun. The (-) charged electrons are found inside (+) dough (pudding)
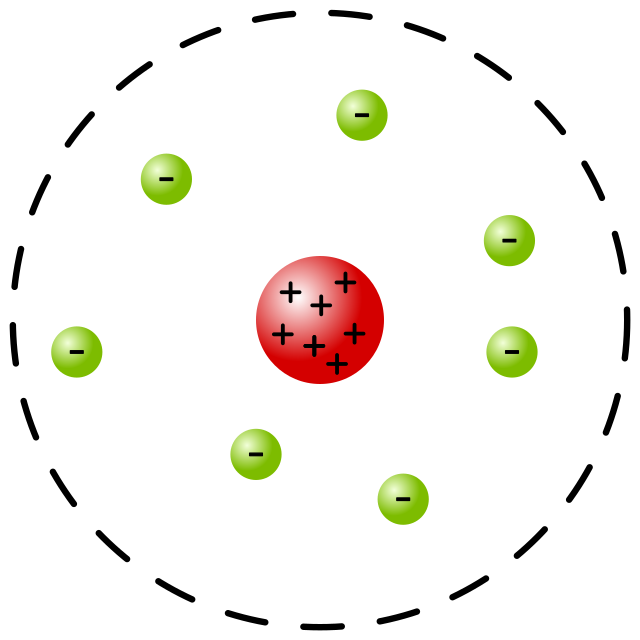
Rutherford 1911
Atoms have (+) particies in the center, and are mostly empty space. (+) particles
called protons, the center of atoms called nucleus (gold foil experiment)
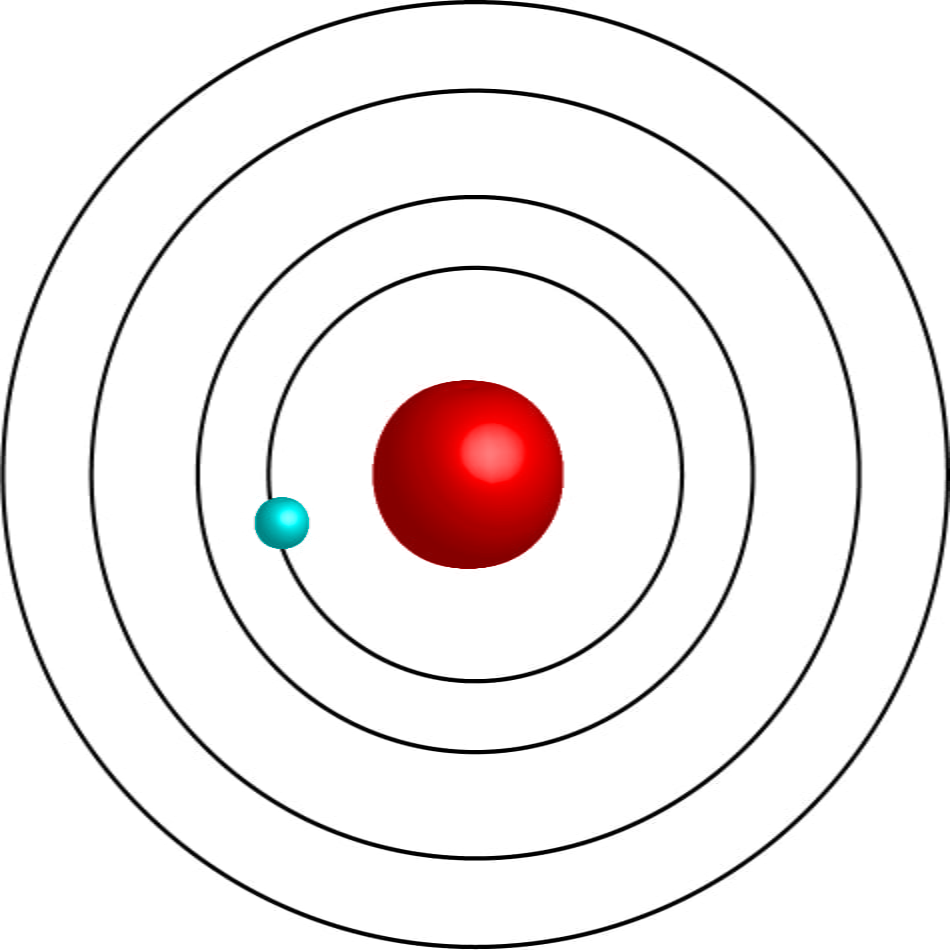
Bohr 1913
improved on Rutherford's model.
He proposed that electrons move around
the nucleus in specific layers, or shells.
Every atom has a specific number of electrons shell
Chadwick 1932
Worked with Rutherford to discover particles with no charge called neutrons. Neutrons are also found in the nucleus
Democritus
model

Thomson
model
Rutherford
model
Bohr
model