W3L5: Isomerism in organic chemistry
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
Structural isomerism
Isomers explain why there are so many different carbon compounds: a lot are variations with the same formula
Structural isomers are compounds with the same molecular formula, but atoms are bonded in different orders. Aka configurational isomers
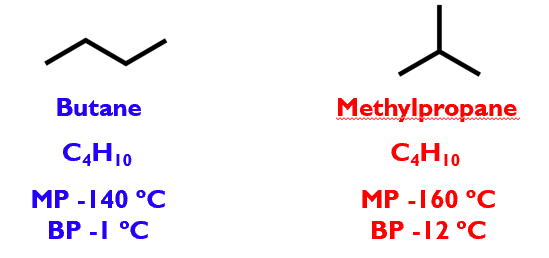
These are structural isomers of one another: contain the same atoms, but butane is linear, methylpropane is branched
Different structures BUT different physical properties
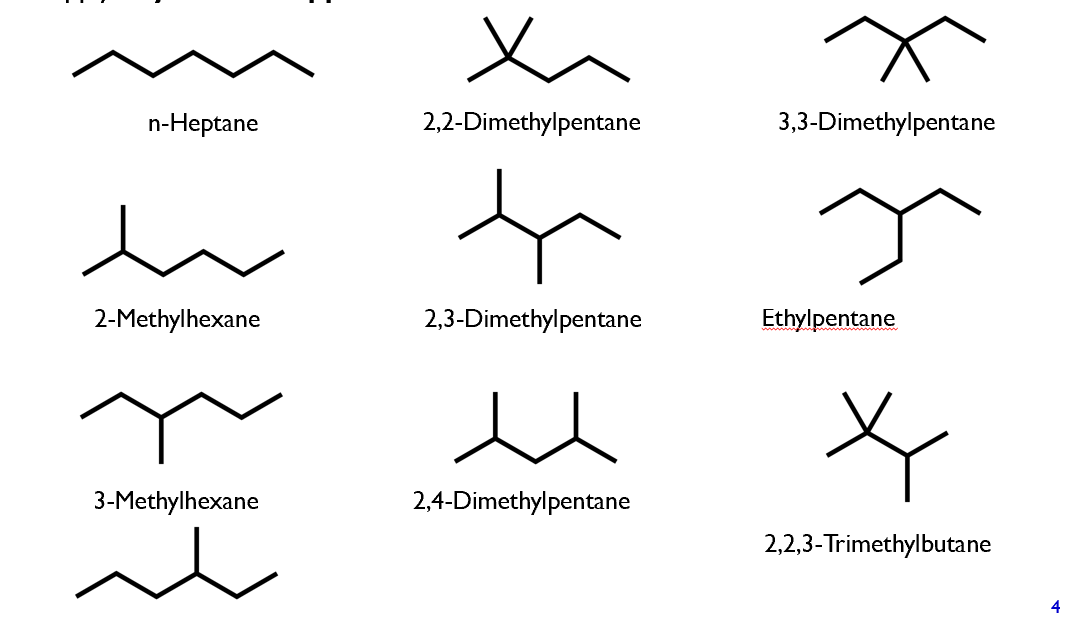
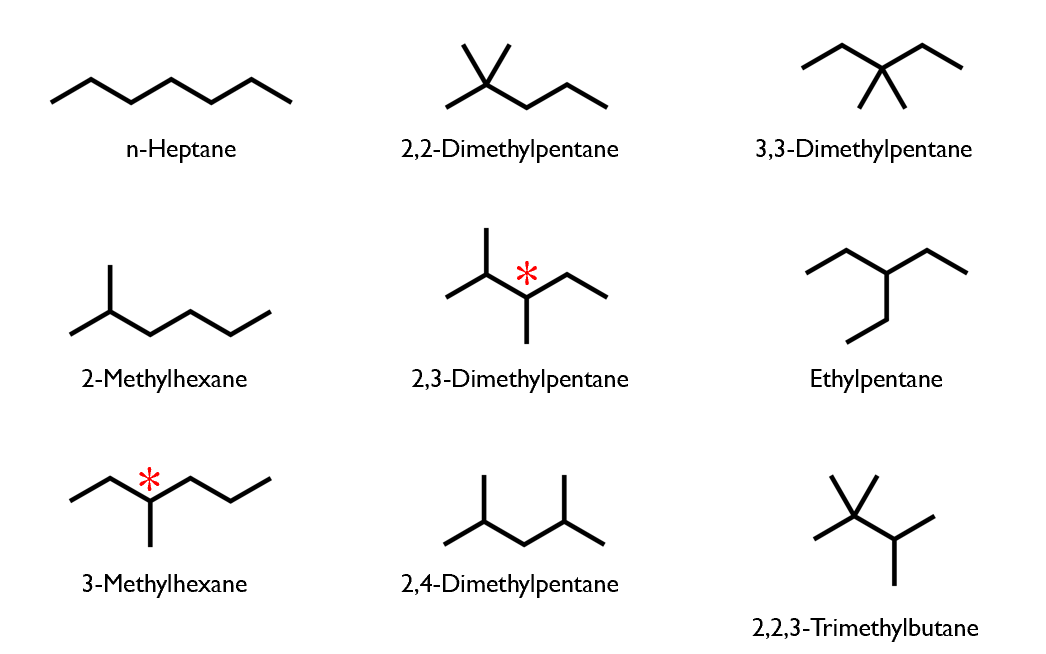
Structural isomers of heptane: C7H16
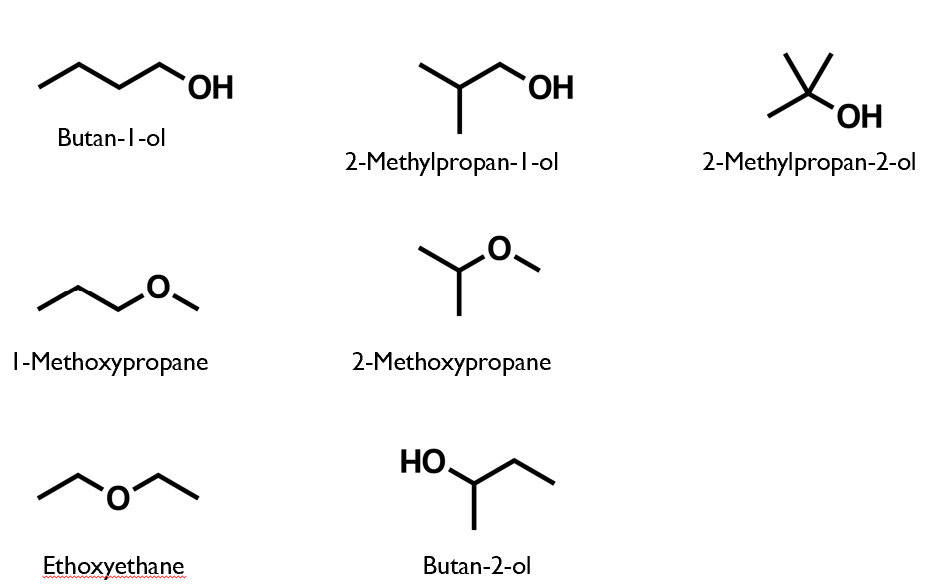
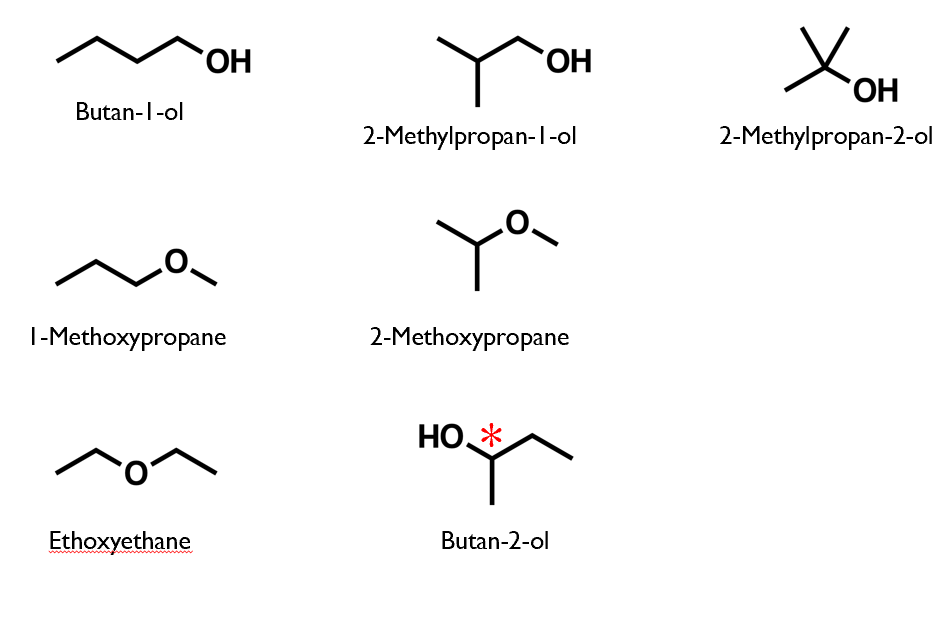
Structural isomers of C4H10O
Stereoisomerism
Stereoisomers are compounds with identical formulae, atoms bonded in the same order,
but in a different spatial arrangement
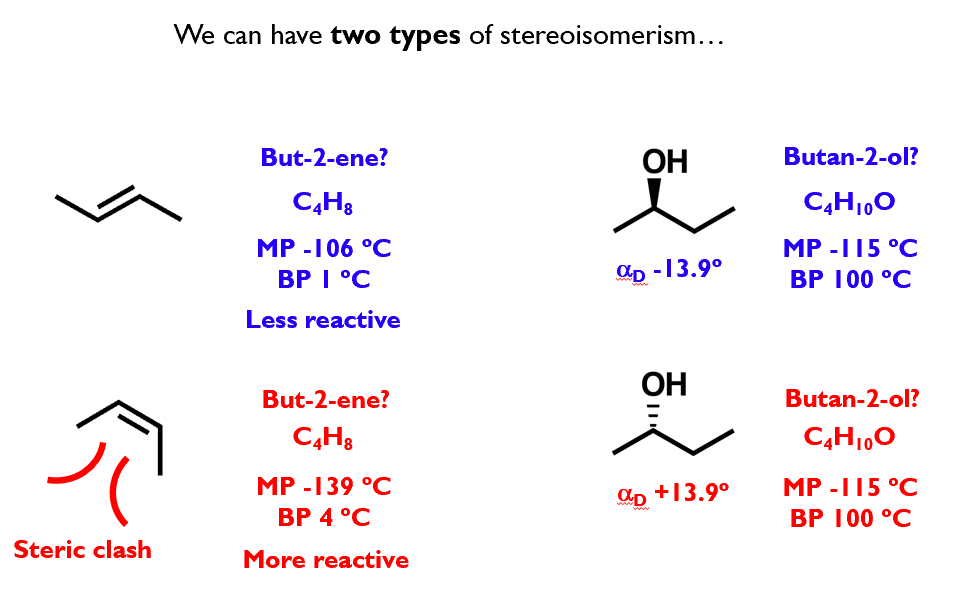
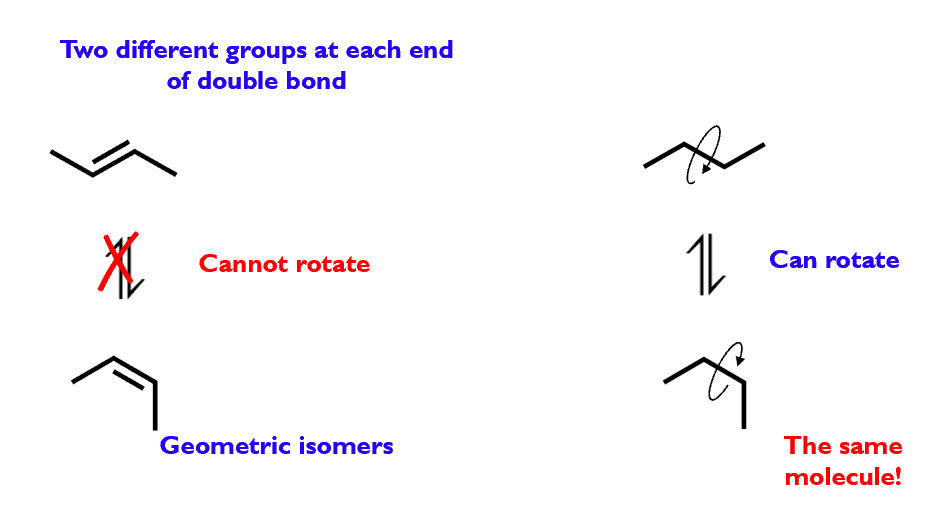
Geometric isomerism
Geometric isomers are stereoisomers that arise due to restricted rotation within a molecule
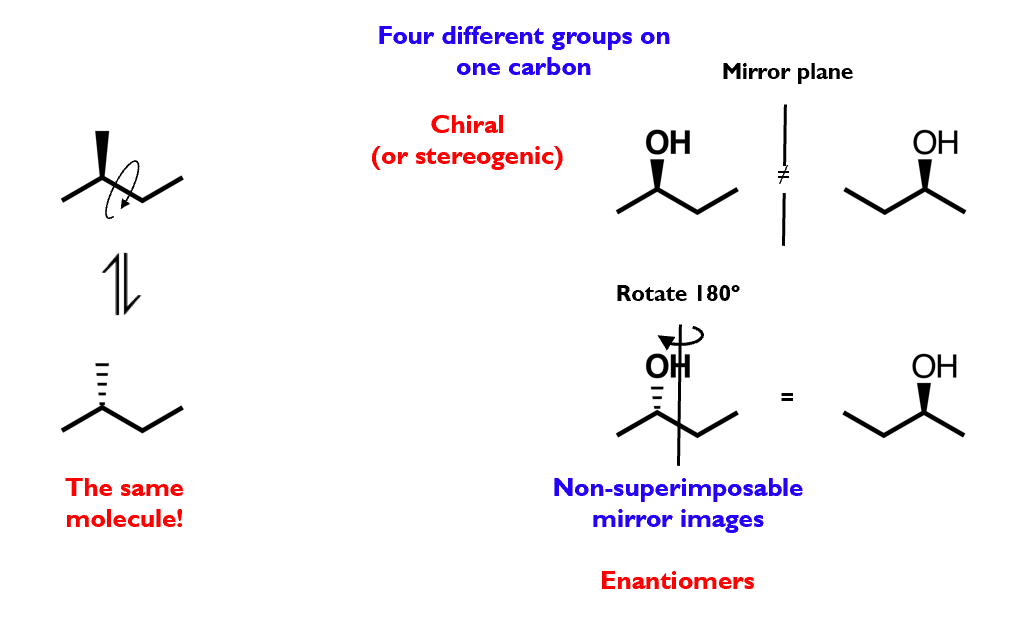
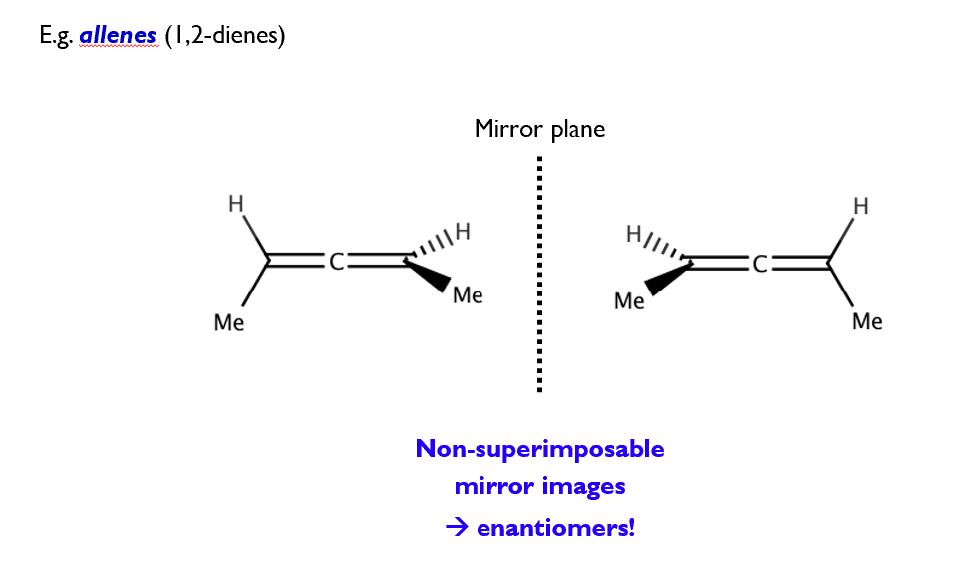
Optical isomerism
Optical isomers are stereoisomers that arise due to reflectional symmetry
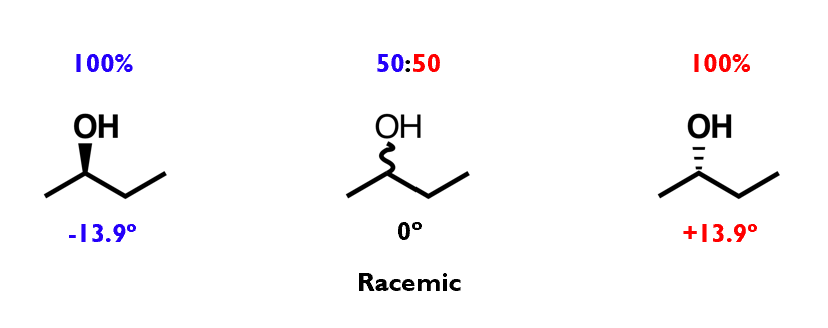
Optical activity
Chiral molecules rotate plane-polarised light: this is called optical activity
A mixture that contains more of one enantiomer than the other is optically active
ALL isomers of C7H16
ALL isomers of C4H10O
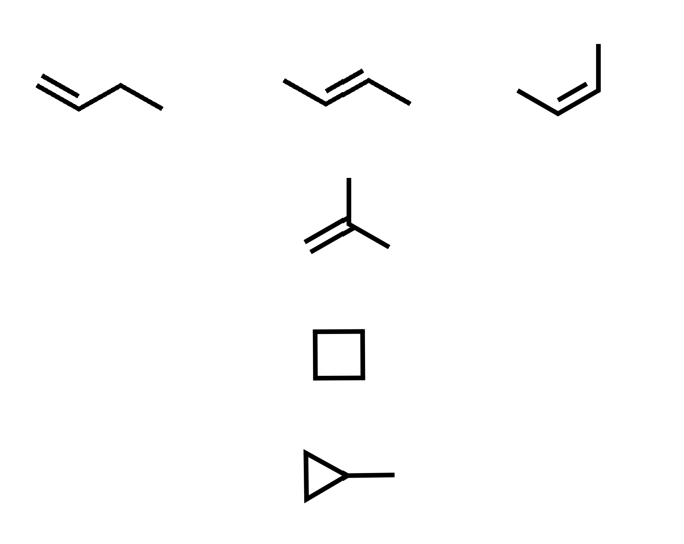
ALL isomers of C4H8
We have partly discussed these already: there is one DBE here
we need to consider double bonds and rings
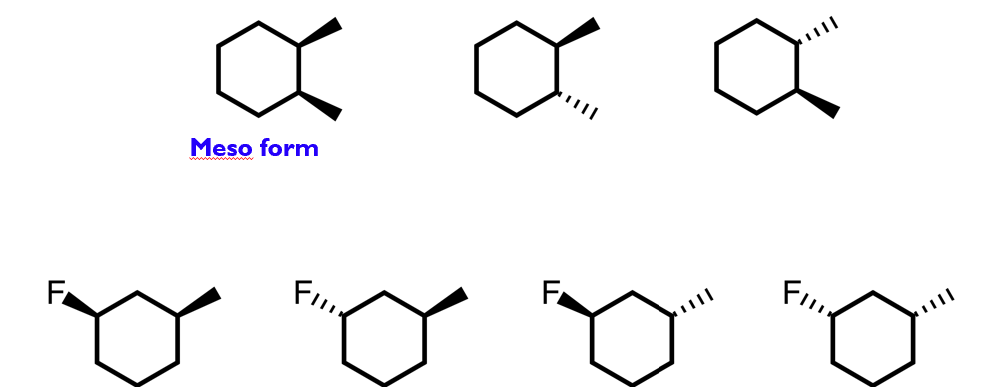
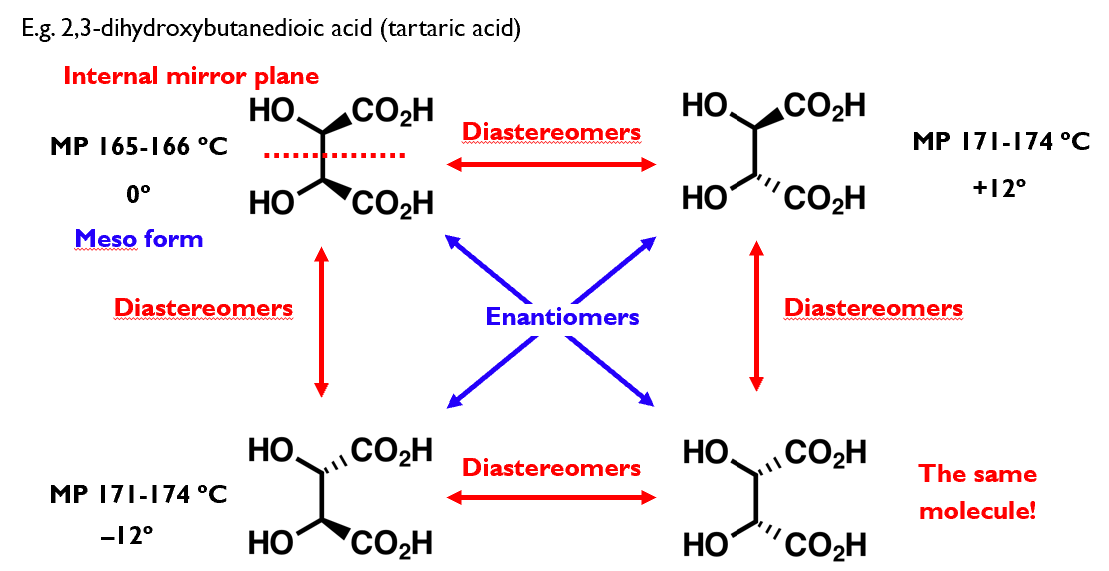
Multiple stereogenic centres
Each additional stereogenic centre generates additional isomers
Generally speaking, n stereogenic centres gives 2n stereoisomers.
The exception to this is when meso forms exist:
Molecules with no stereogenic centres, which do display optical isomerism
Rings can display both geometric and optical isomerism