Fuels Theory
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
What is fuel?
A fuel is a substance that undergoes combustion with oxygen to release energy.
Most fuels are organic (derived from living things)
Normally for combustion to occur you have to ignite the fuel.
How is food fuel?
Foods provide fuel but are not combusted.
Carbohydrates are broken down in the body and produces glucose.
Glucose is then used during respiration to release energy.
How can fuels combust?
Most fuels contain carbon, hydrogen and sometimes oxygen.
A general word equation for the combustion of a fuel can be written as:
Fuel + Oxygen —- Carbon dioxide + Water
How do fuels come from crude oil?
Crude oil is made up mainly of hydrocarbons (a compound made only of carbon and hydrogen).
Most hydrocarbons in crude oil are alkanes.
These are saturated hydrocarbons. This means there are only single bonds.
What are alkanes?
A type of hydrocarbon.
General formula (CnH2n + 2)
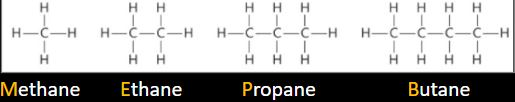
The first four hydrocarbons are…
Methane: (CH4)
Ethane: (C2H6)
Propane: (C3H8)
Butane: (C4H10)
Pentane, Hexane, Heptane, Octane, Nonane, Decane
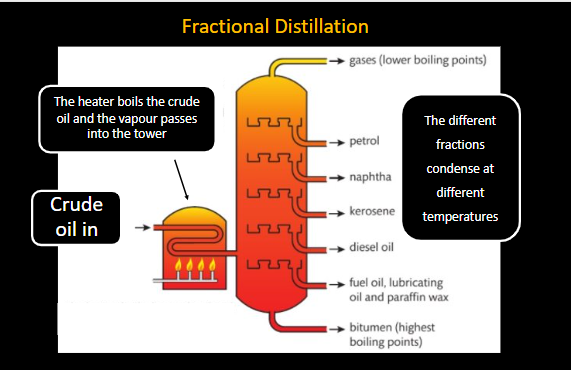
What is Fractional distillation?
Crude oil itself is not particularly useful, but it can be seperated into useful fractions by the process of fractional distillation.
This occurs by…
The crude oil goes in the fractional distillation
The heater boils the crude oil and the vapour passes into the tower
The different fractions condense at different temperatures.
How does the length of fractions affect the boiling point?
As the length of the hydrocarbons chain increases, there are more intermolecular forces between the chains.
More energy is required to break these forces, explaining the increase in boiling point.
What are three examples of fractions?
Petroleum gas
1 to 4 number of carbons
more than 20oc bp
gas
Disel (gas oil)
13 to 20 number of carbons
220 to 350 bp
liquid
Paraffin wax
29 to 30 carbon
less than 400 bp
solid
As the fractions number of carbons increase what happens to their colour, viscosity, ease of ignition and sootiness of flame.
Colour = Darkens as number of carbons increase
Viscosity = Increases as number of C atoms increases.
Ease of ignition = Decreases as number of C atoms increases
Sootiness of flame = Increases as C increases
How are alchohols used as fuels?
Alcohols are made up of carbon, hydrogen and oxygen atoms.
They have a general formula CnH2n+1OH.
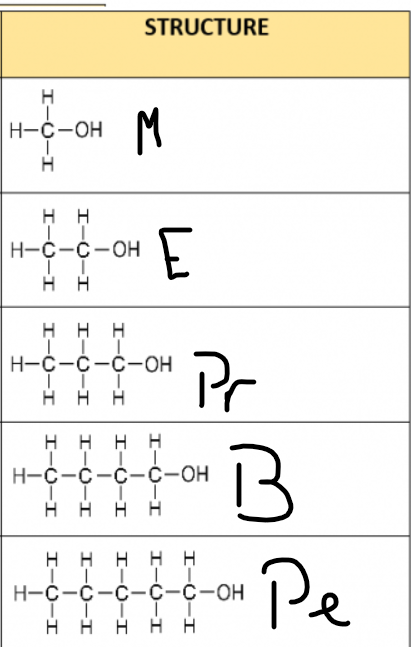
What are the formula of the first 5 alcohols..
Methanol: CH3OH
Ethanol: C2H5OH
Propan-1-ol: C3H7OH
Butan-1-ol: C4H9OH
Pentan-1-ol: C5H11OH
What are the advantages of alcohols?
Alcohols with small numbers of carbon atoms burn more cleanly than liquid hydrocarbon fuels as petrol.
They can also be obtained from renewable energy sources, so can preserve crude oil and natural gas.
How do alcohols burn?
Ethanol can be produced by fermentation and can be used as a fuel for cars, either on its own or mixed with petrol to make gasohol.
Burning ethanol produces carbon dioxide and water.
ethanol + oxygen —- carbon dioxide + water.
C2H5OH + 3O2 —— 2CO2 + 3H2O
What are the Environmental impact of burning alcohols?
Even though carbon dioxide is produced when ethanol is burned, it is considered carbon neutral.
This is because the plants used to make ethanol use carbon dioxide from the air in phototsynthesis to grow.
What are the Hazards of Fuels?
Toxicity (Skull): Some fuels are toxic to humans. As little as 10 cm3 of methanol can cause blindness, coma or even death.
Flammability (Fire): Fuels must be ignited before being used. Careless use could lead to a fire, so fuels must be appropriately labelled.
Explosive (Explosion): If gaseous fuels or vapour fuel is released into the air, a spark is likely to cause an explosion.

What Pollution can Complete/Incomplete Combustion cause?
Complete Combustion: When burning fuels, there must be a plentiful supply of oxygen; the only products are carbon dioxide and water. (Blue flame)
Incomplete Combustion: If there is limited oxygen, carbon monoxide and unburnt hydrocarbons may be released into the atmosphere. (Orange flame)

What does Carbon monoxide gas affect? (Pollution)
It is a type of pollution.
Carbon monoxide gas is toxic. It is colourless and odourless.
It binds to haemoglobin more readily than oxygen. If it is inhaled, it means that oxygen will not reach the body’s tissues.
What does Particulates affect? (Pollution)
It is a type of pollution.
Soot is carbon from incomplete combustion.
It can cause respiratory problems, and even global dimming.
What does Sulfur Impurities affect? (Pollution)
It is a type of pollution.
Coal, crude oil and natural gas all contain sulfur as an impurity.
When sulfur burns it produces sulfure dioxide. This can cause respiratory problems, and also causes acidic rain.
What are the Units of Energy?
Calorie - energy needed to raise the temperature of 1g of water by 1oc.
Joule - energy supplied when a force of 1N moves 1m.
Kilowatt hour - energy used by a 1KW appliance in 1 hour.
Energy values are usually given in kJ or kcal to avoid using large numbers
e.g. a packet of crisps has 150 kcal or 150,000 calories.
How can we find the Heat Energy released by fuels?
We can find the heat energy supplied by a fuel using the equation:
E = mc0
E = energy transferred in joules, J
m = mass of water in Kg
c = specific heat capacity of water J/Kg oc
0 (‘theta’) = temperature change in degrees Celsius, oc.
How do convert KJ to J and Kg to g?
11 KJ = 11000J (x by 1000)
11j = 0.011KJ (/ by 1000)
11kg = 11000 g (x by 1000)
11g = 0.011Kg (/ by 1000))
What does Specific Heat Capacity mean? (C)
The energy required to raise the temperature of 1g of a substance by 1oc.
Once you have calculated the energy, you can work out the heat energy per kg.
For a pure compound you can find the energy supplied in kJmol-1.
What is the calculation for Heat Energy per mole?
Heat energy per mole (Jmol-1)
= Heat energy x relative moleular mass / Mass of fuel burnt.
What happenes to the Bond Energies when fuels burnt?
When a fuel is burnt, bonds in the fuel and oxygen molecules are broken, this uses energy.
Energy is released when bonds form in the reactants.
More energy is released by bonds forming than is used when bonds are broken, meaning the reaction is exothermic.
The bigger the chain, the more heat energy that is released.
Which is more effective: Incomplete or Complete combustion?
Incomplete combustion is less efficient, and less energy is released when bonds form in carbon monoxide or carbon.
What happens to the alcohols the further you go?
Each alcohol has one more CH2 group than the previous.
This means 1 more C-C bond and 2 more C-H bonds.
This will require more energy to break it. But there will be more C=O bonds in CO2 and more H-O bonds in H2O forming.
The energy released when forming these bonds is greater than the energy required to break so more energy is released as the carbon chain increases.
MEXO (Make bonds - exothermic)
BENDO (Break bonds - endothermic)