Chemistry - Graphite
1/5
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
Covalent network bond
bonding elements share electrons but in a 3-D crystal lattice
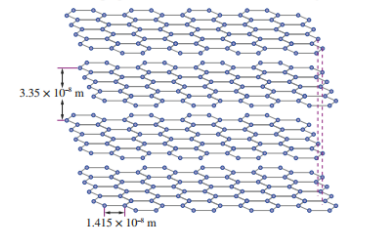
Graphite structure
-In graphite carbon forms 3 bonds
-Weak dispersion forces between layers
High MP
-due to strong covalent bonds between carbons
-High energy to lossen/break
Conductive (known only in solid)
-In graphite carbon forms 3 bonds instead of 4
-Remaining electron is delocalised and able to move between layers
-Due to mobile and charge it conducts
-Only known in physical state and not liquid to due melting point being 3000o
Soft
-Weak dispersion forces connecting layers
-structure is able to move around without breaking the covalent bond, hence soft
Brittle
-Extremely strong covalent bonds between carbons which are directional
-Cannot withstand extreme forces instead will shatter. Therefore brittle