Covalent Bonding and Naming
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
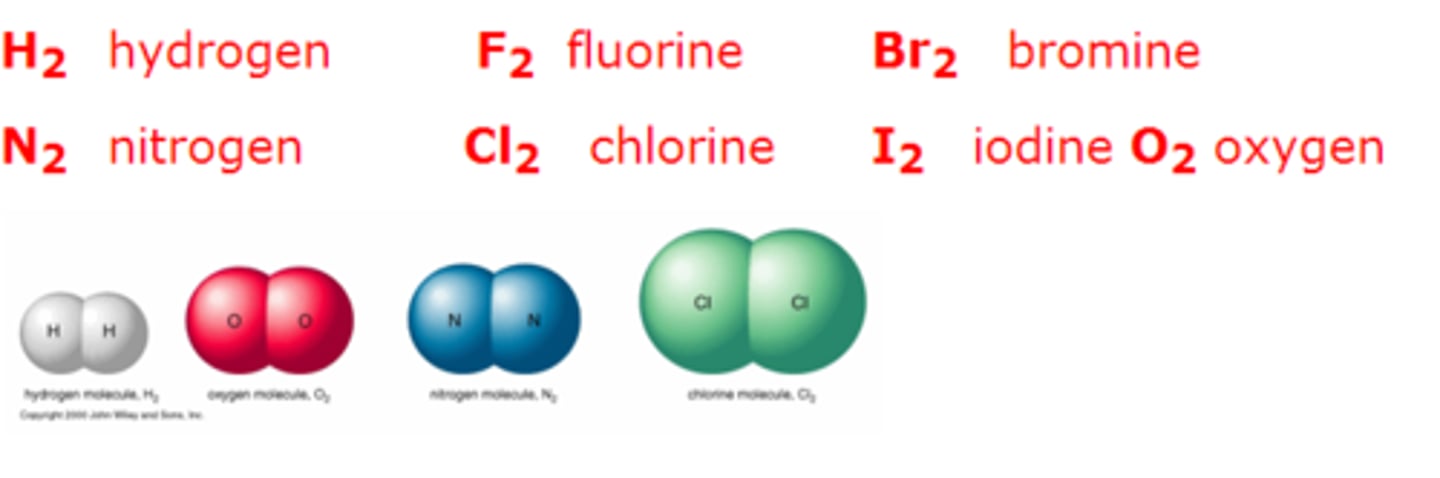
Diatomic molecule
2 atoms of the same element

Diatomic molecules are
H2, N2, O2, F2, Cl2, Br2, I2
Molecule
covalently bonded atoms
Covalent Bond
atoms share electrons, between two nonmetals
single bond
1 pair of shared electrons between 2 atoms
double bond
2 pairs of shared electrons between 2 atoms
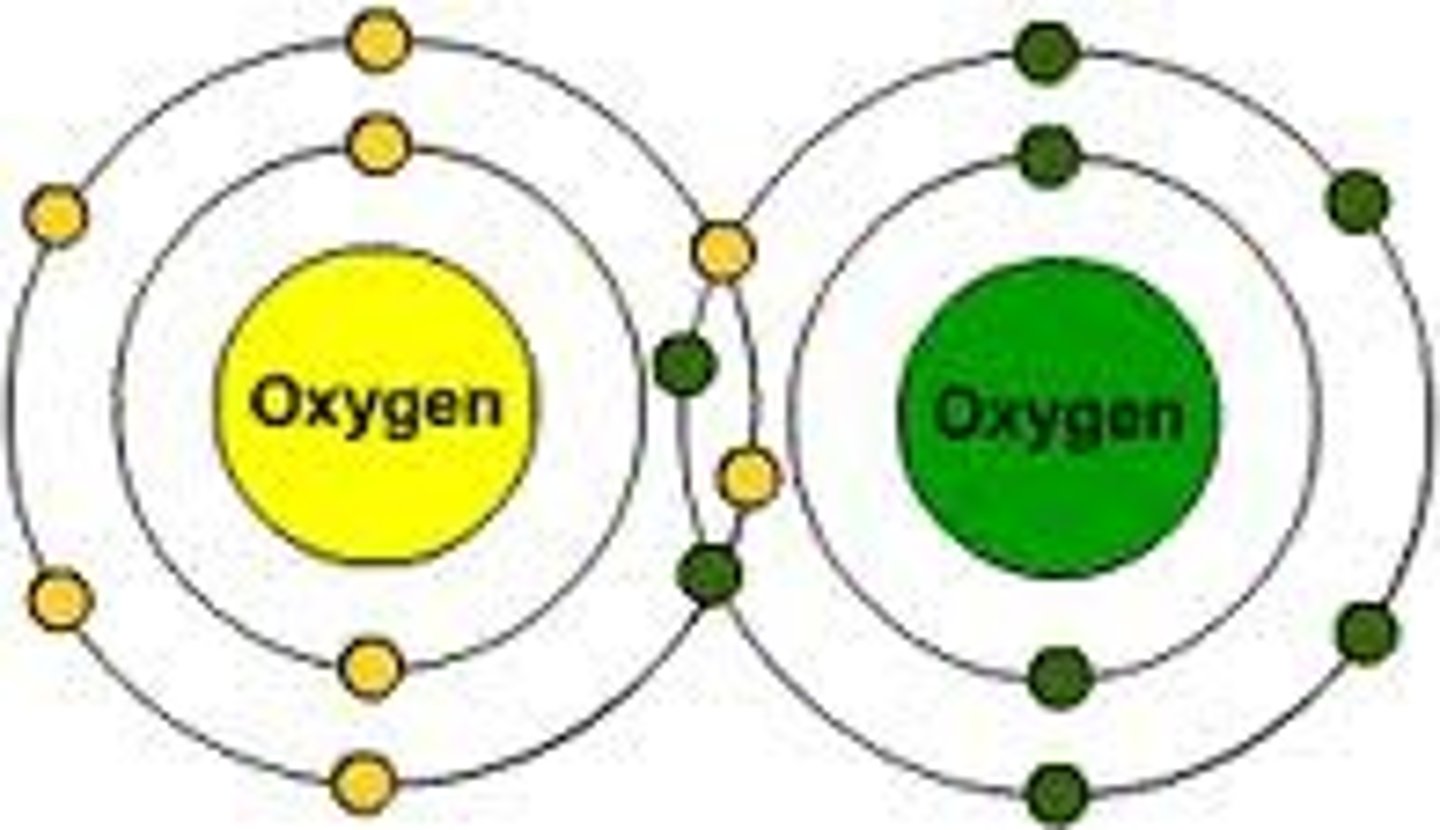
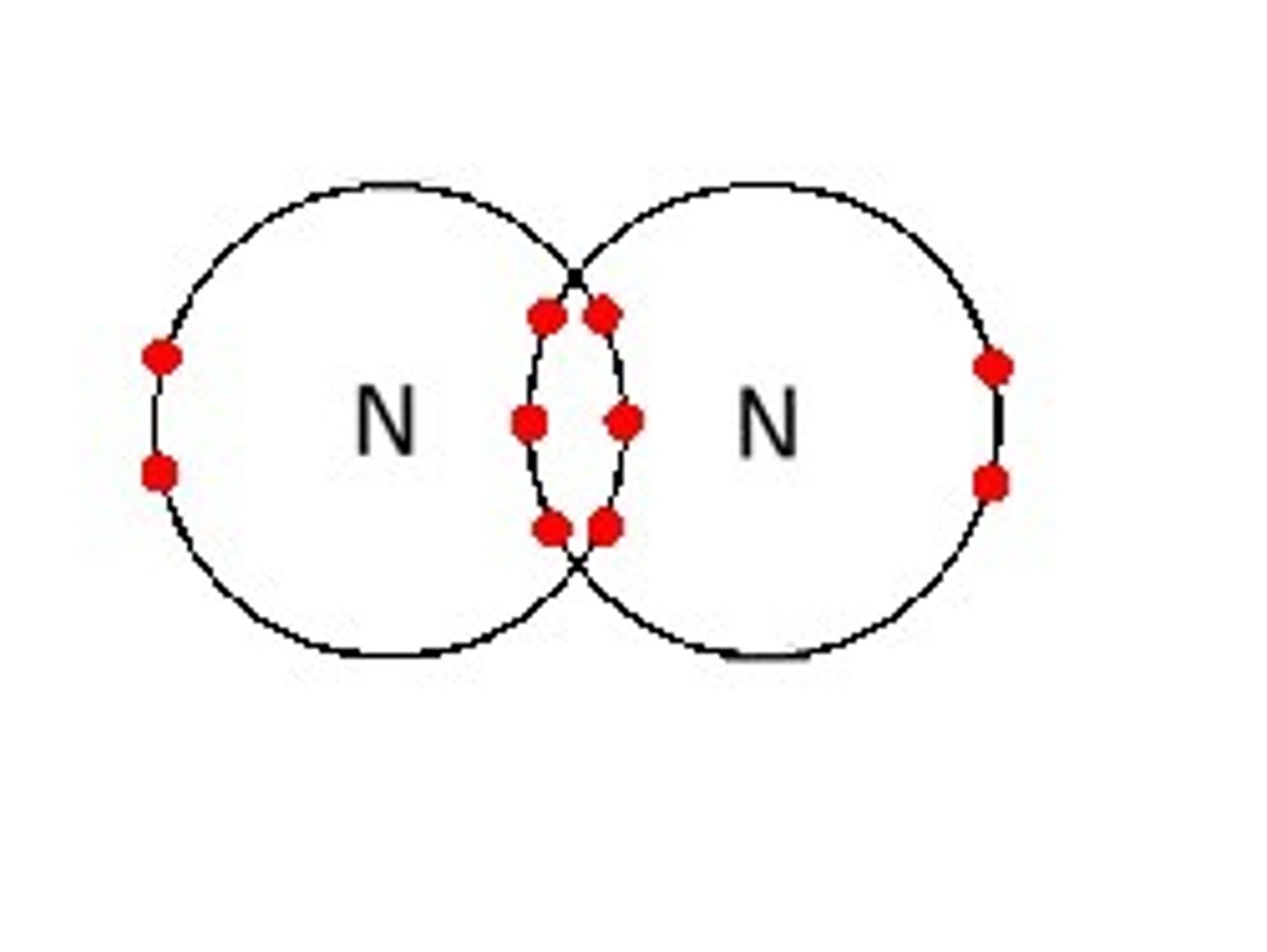
triple bond
3 pair of shared electrons between 2 atoms
Examples of Diatomic molecules
H2, N2, O2, F2, Cl2, Br2, I2

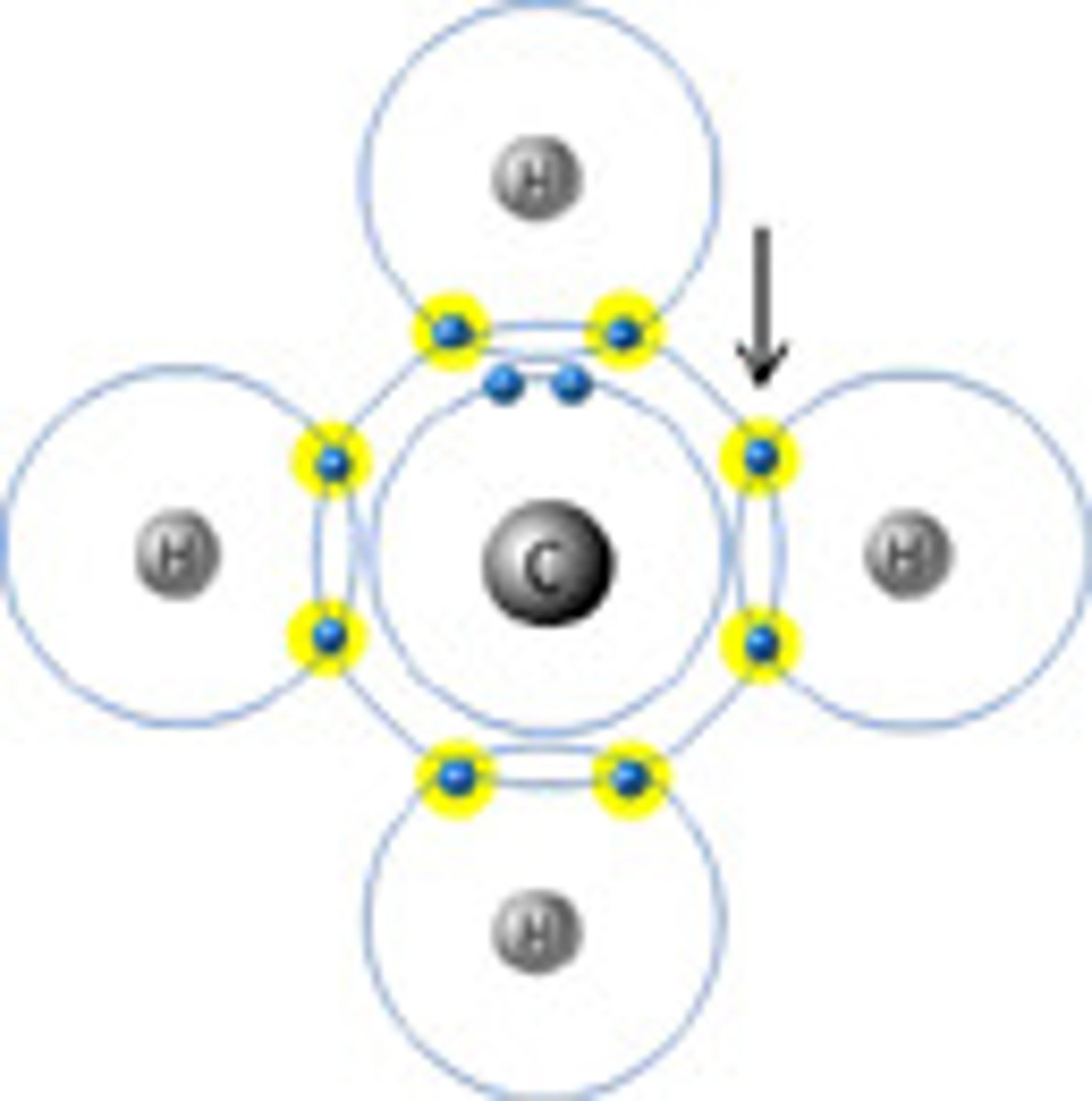
4
Number of bonds CARBON can make
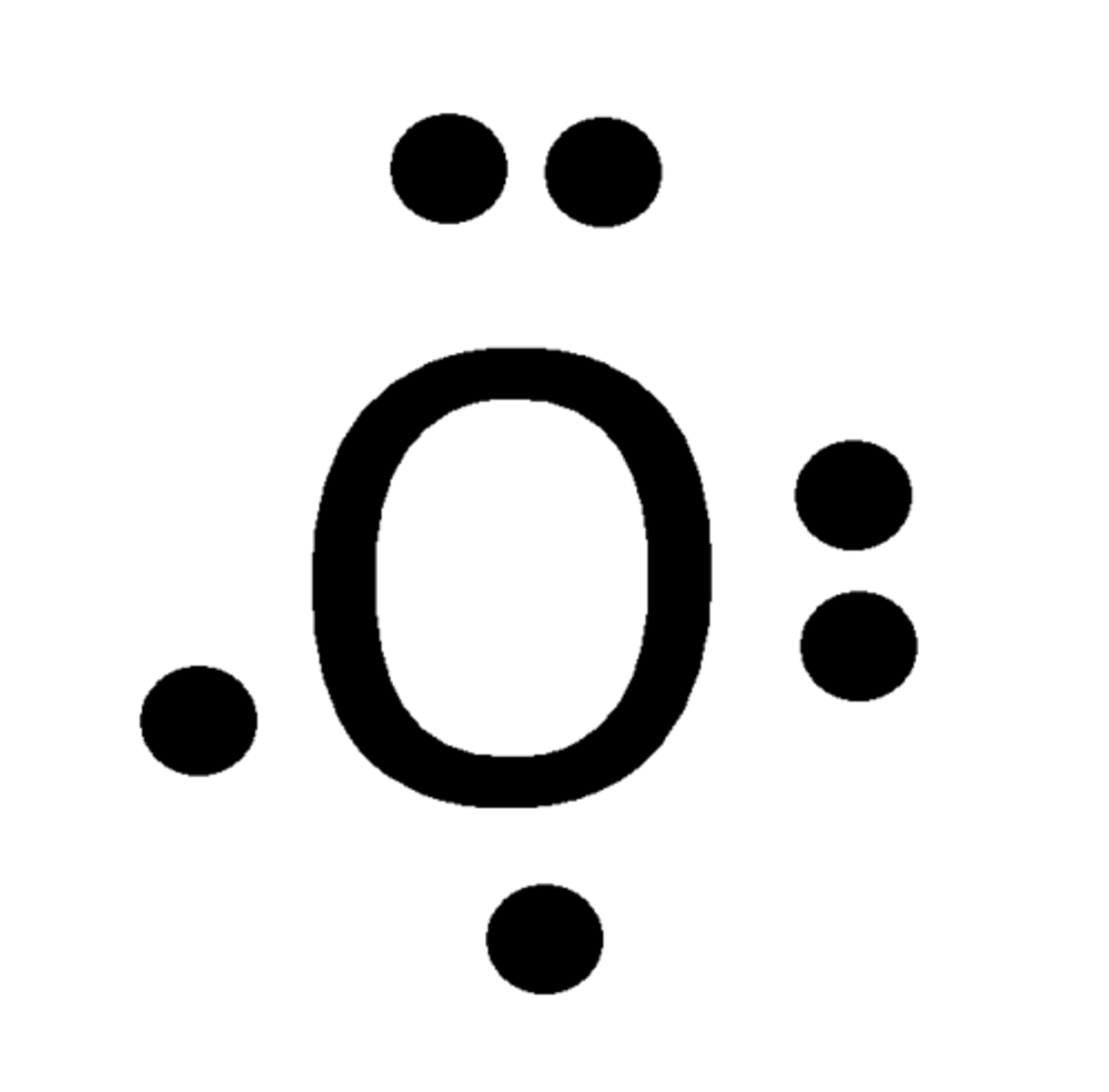
2
Number of bonds OXYGEN can make
3
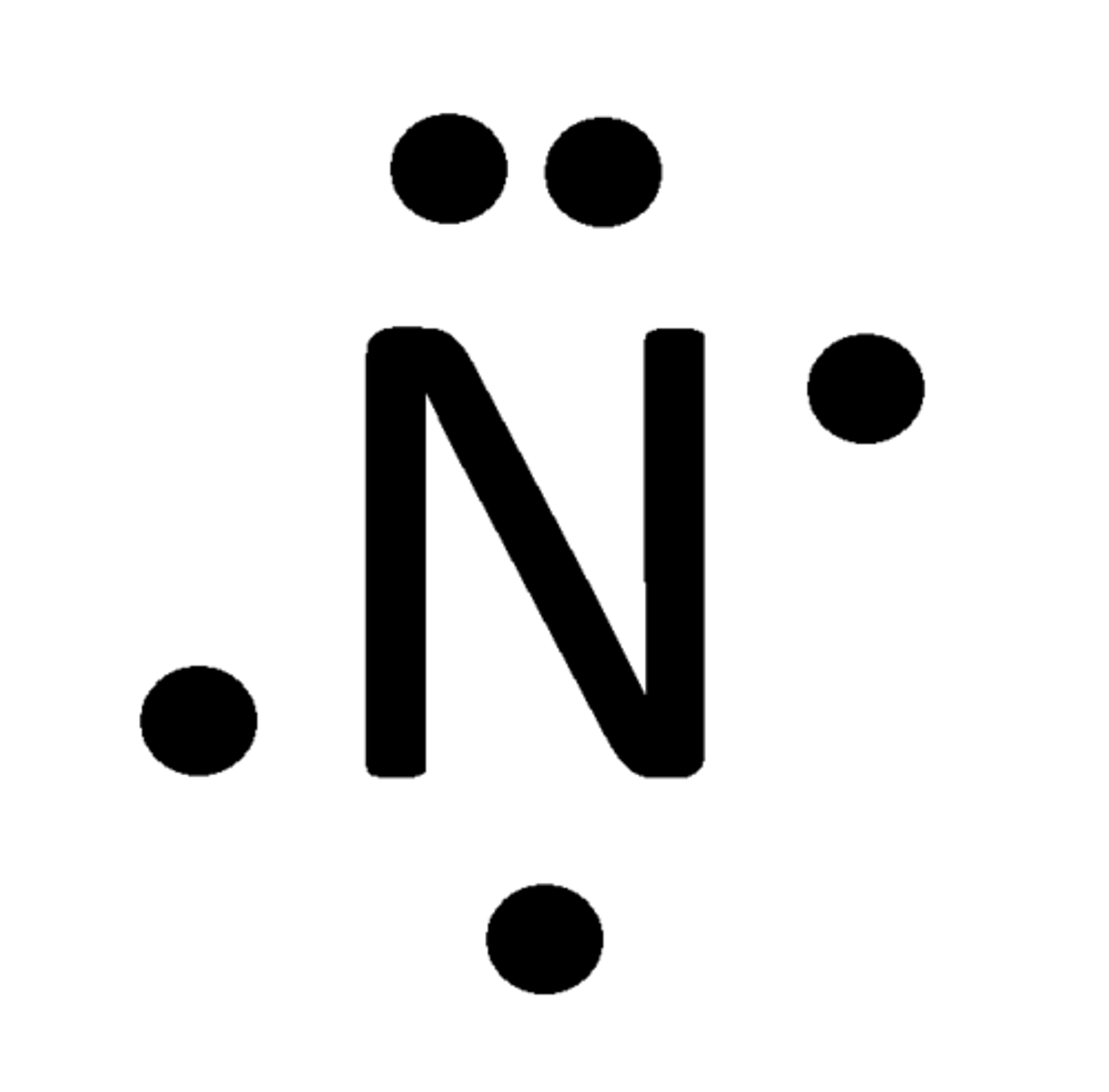
Number of bonds NITROGEN can make
1
Number of bonds CHLORINE can make

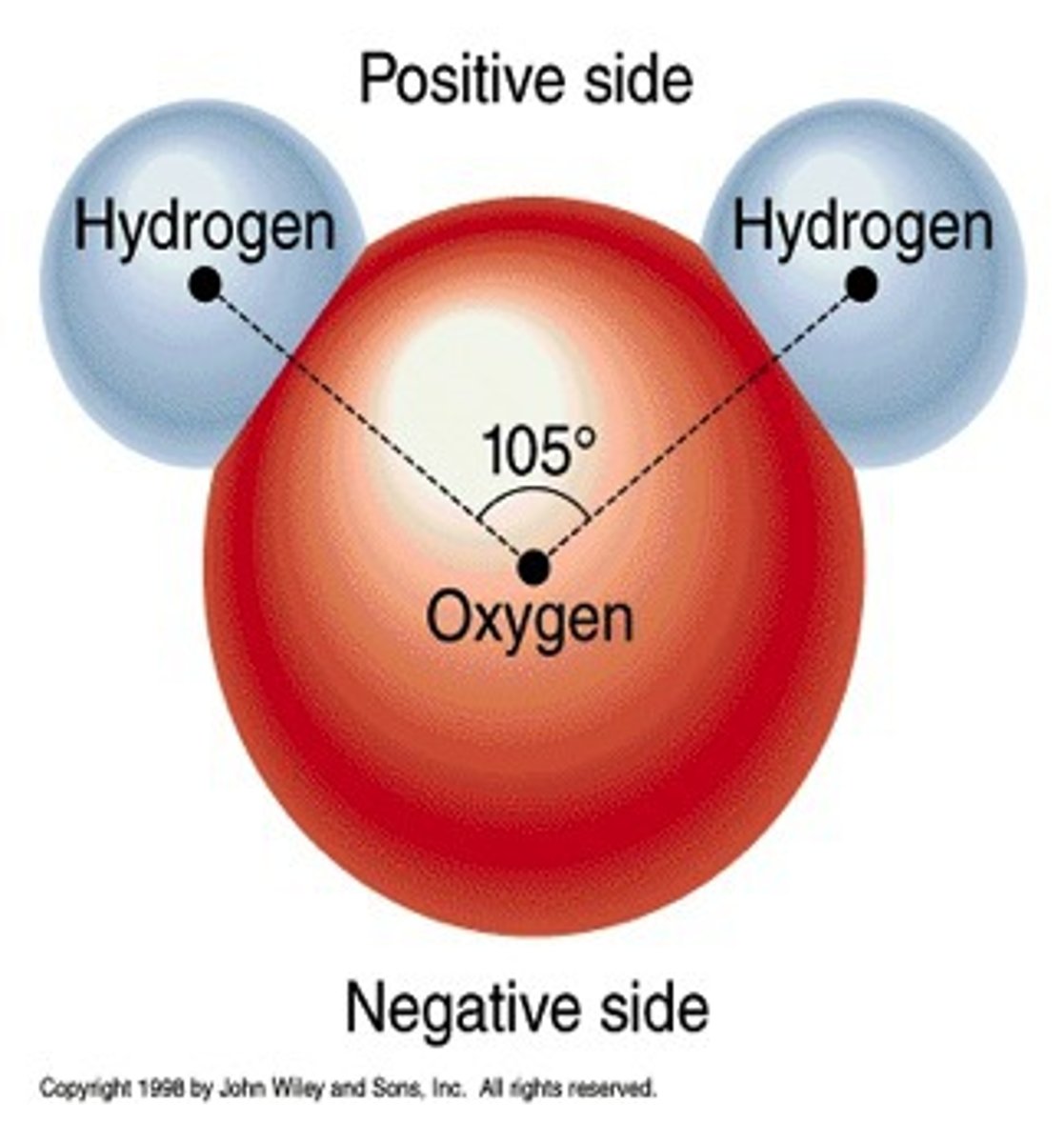
H2O
Dihydrogen monoxide

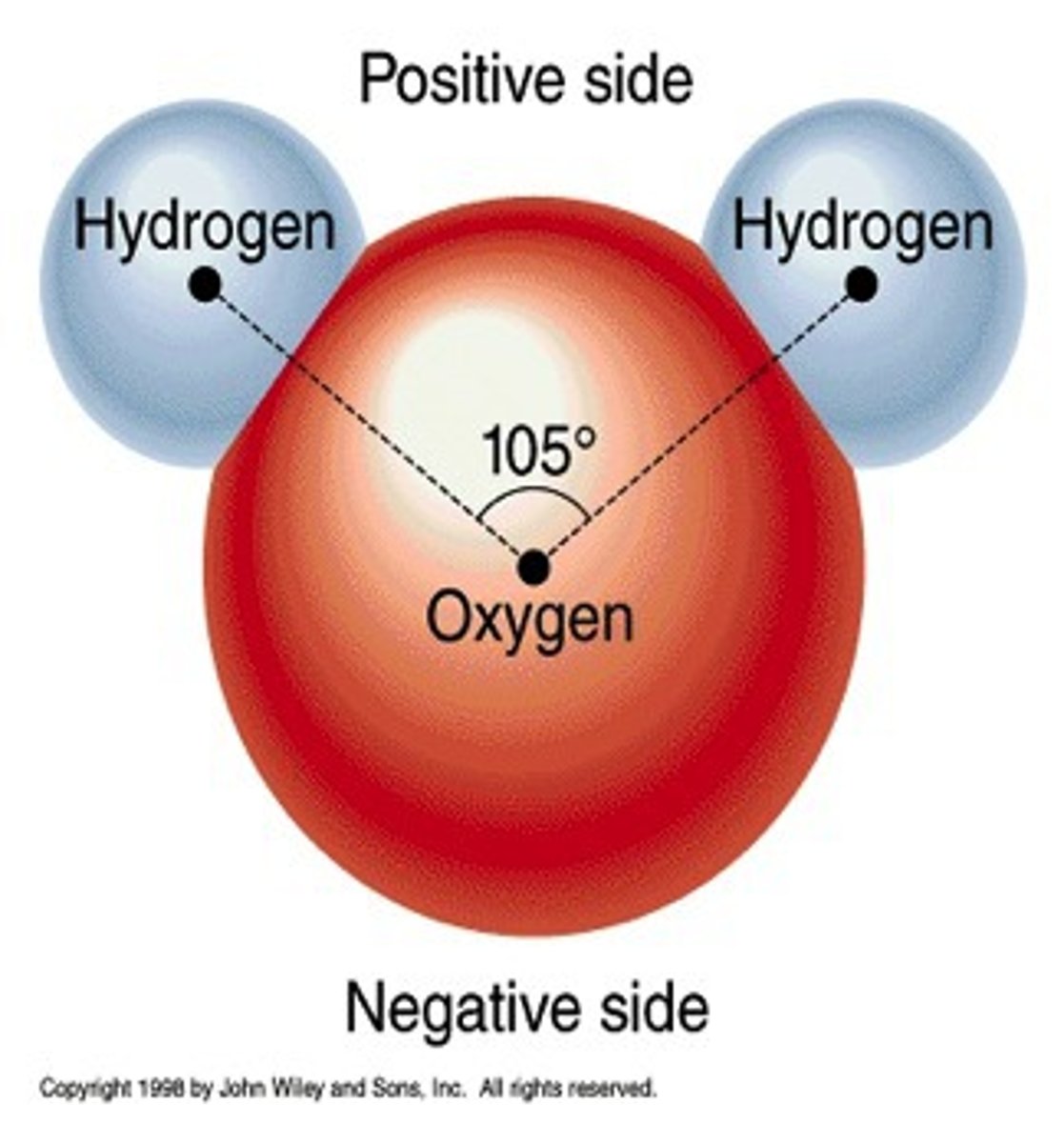
Example of a polar bond
H2O

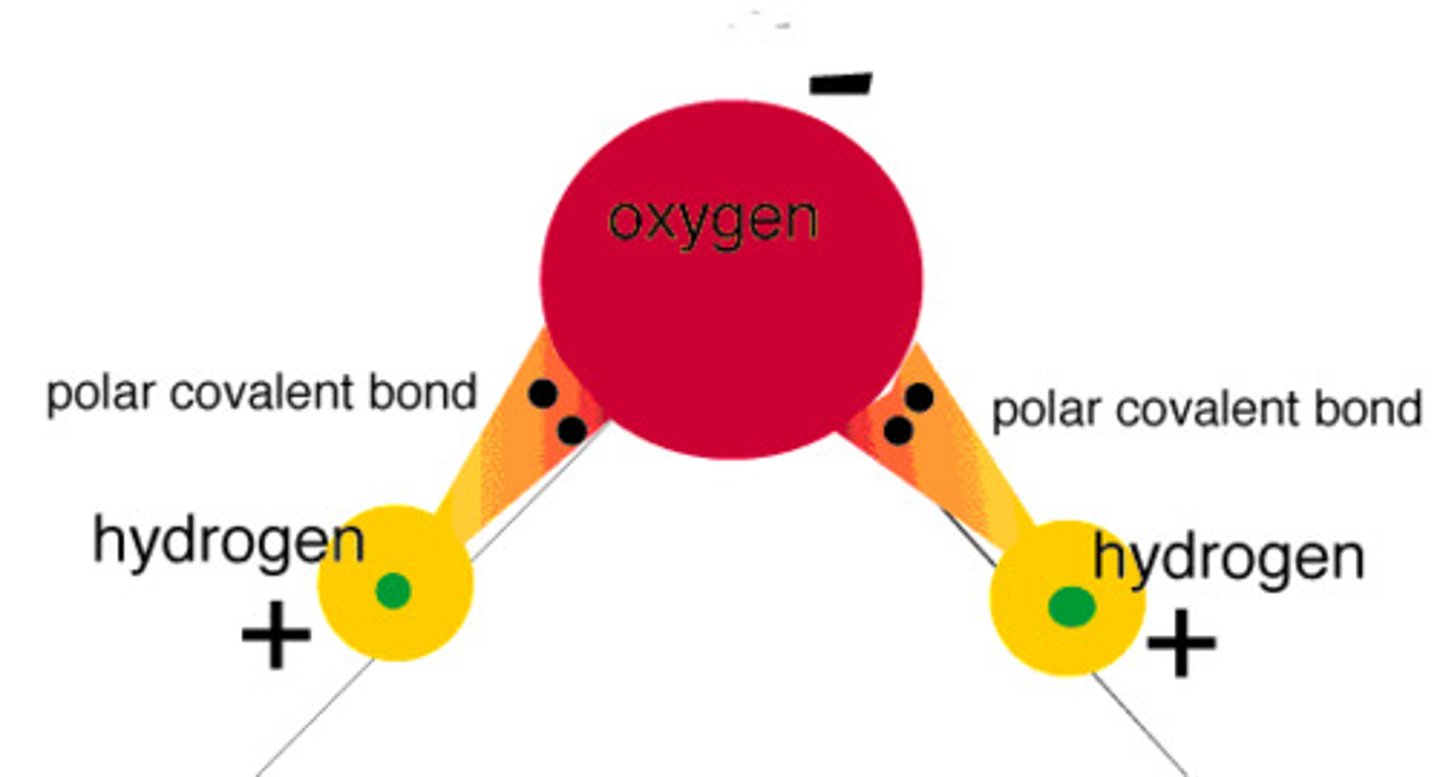
Polar covalent bond
Unequal sharing of electrons in a molecule
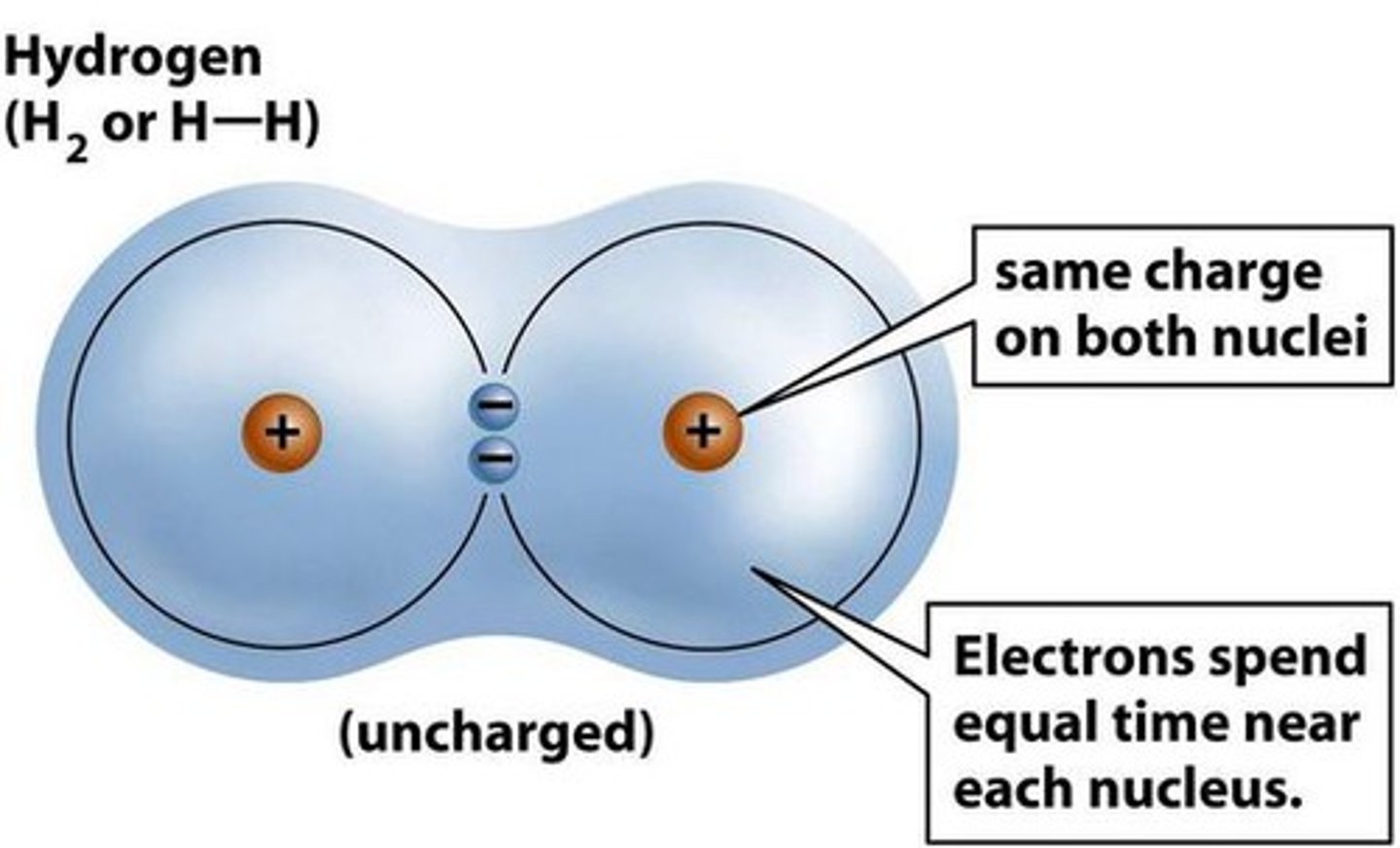
Non-polar covalent bond
Equal sharing of electrons in a molecule
Covalent Molecule Names
Names using prefixes
lone pair
Pair of electrons associated with one atom in a molecule and not involved in bonding.
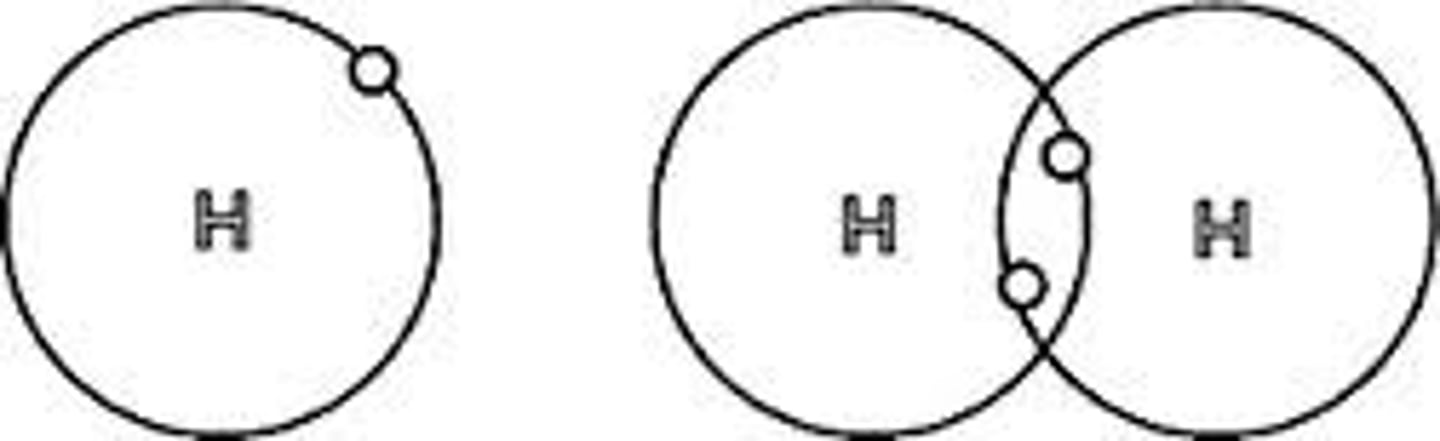
Lewis Dot Structure
diagram of a molecule using dots to represent valence electrons
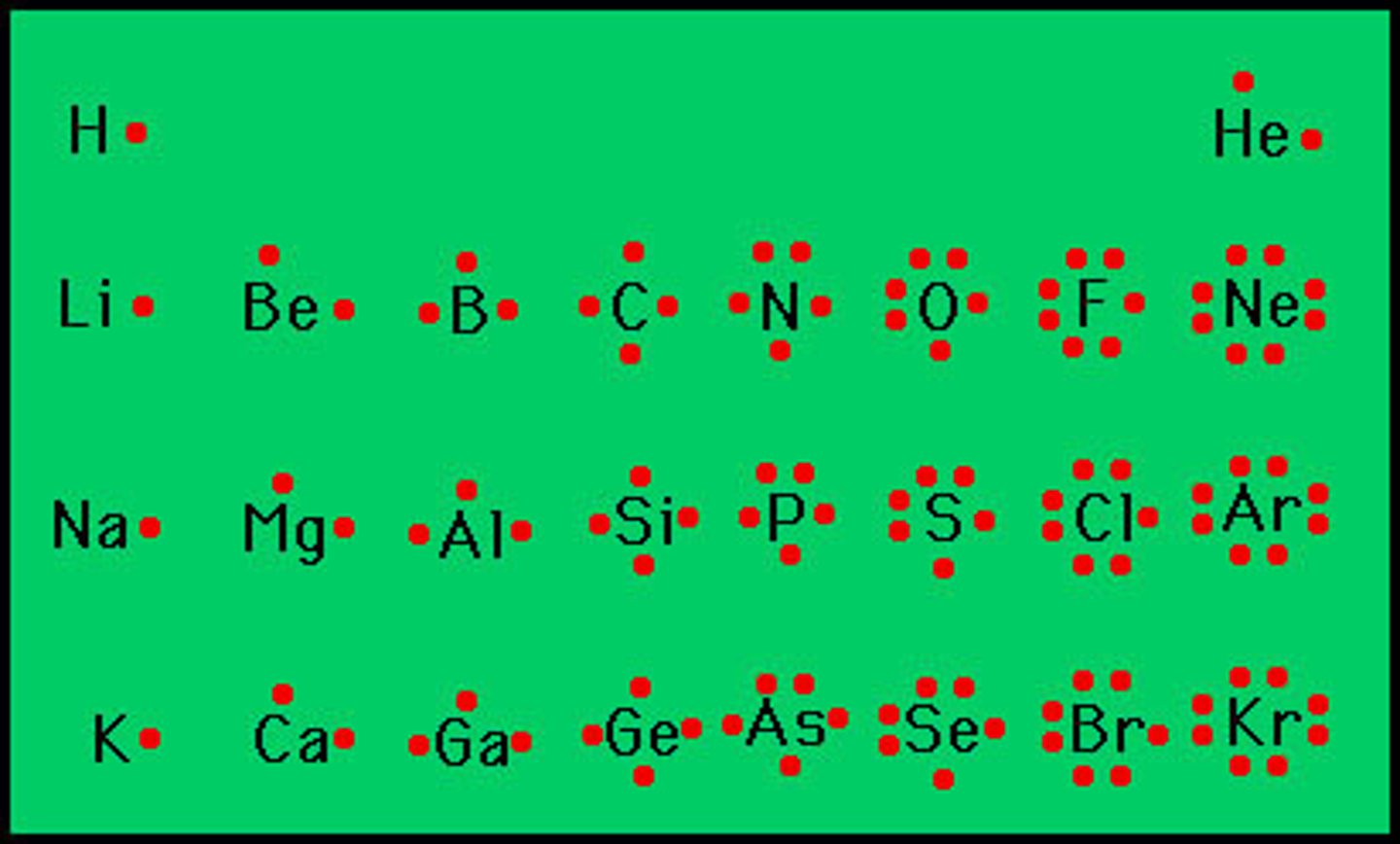
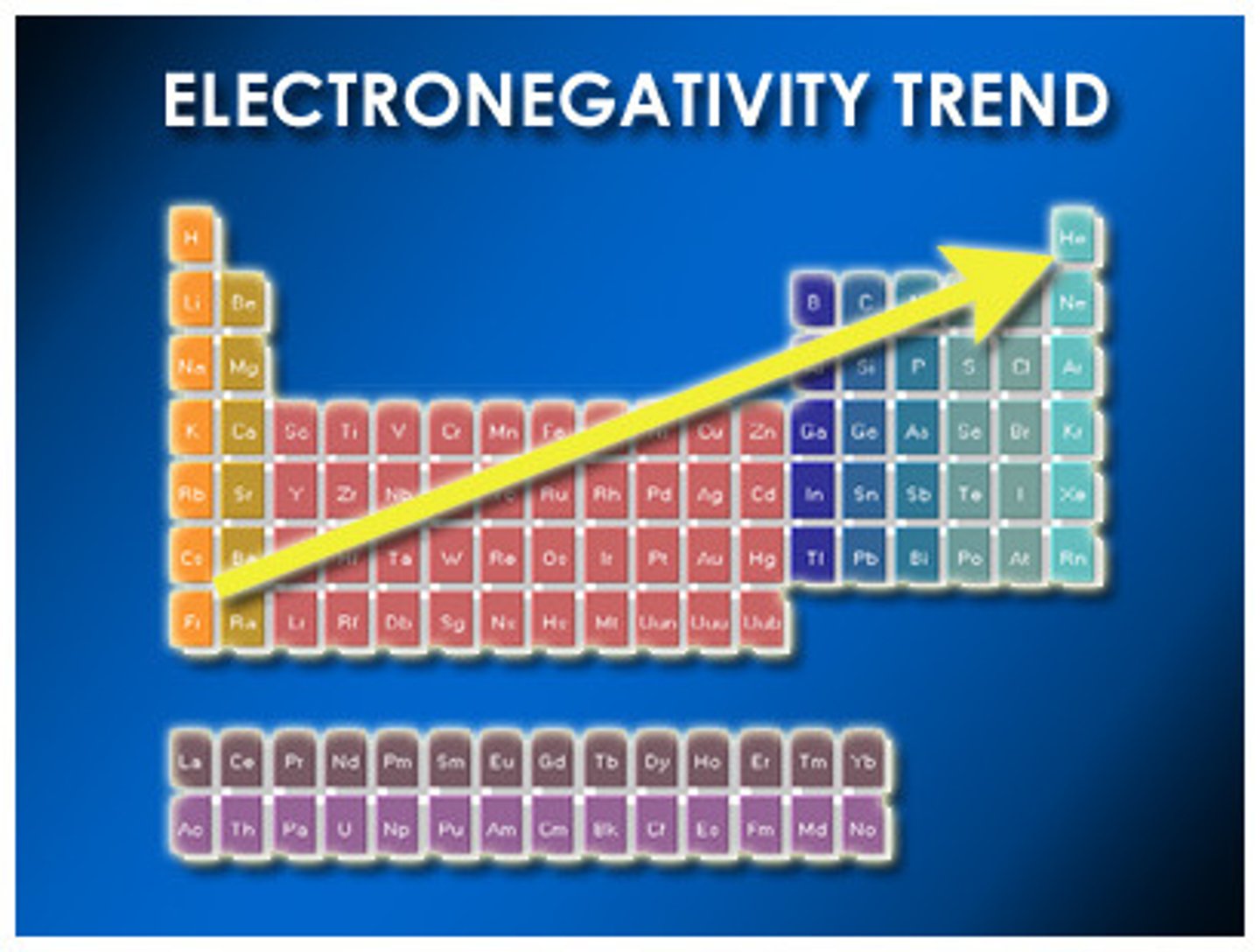
Electronegativity
the ability of an atom to attract electrons when the atom is in a compound
Covalent compound properties
between 2 nonmetals, liquid or gas, not soluble, shared electrons
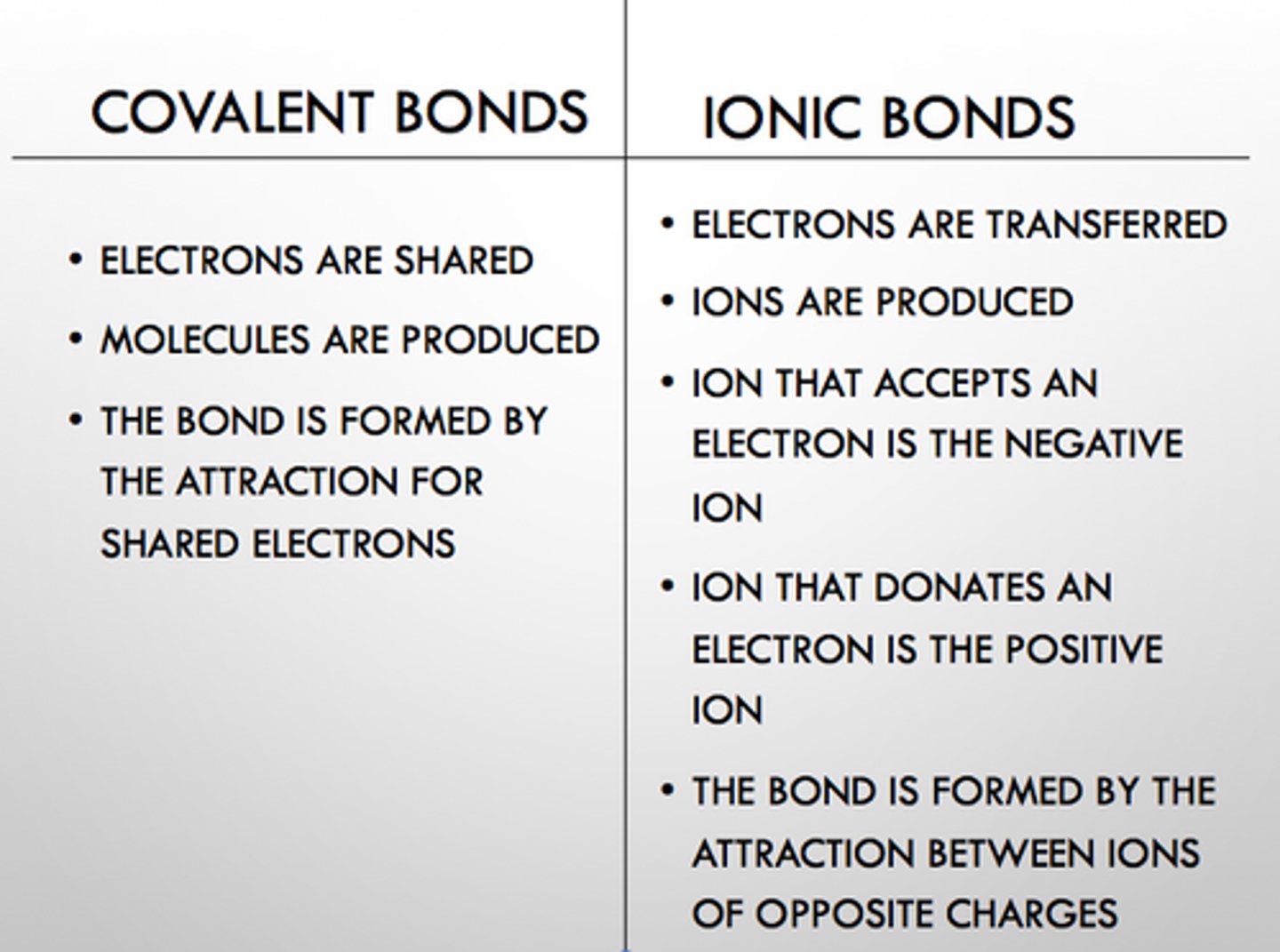
Ionic compound properties
between a metal and a nonmetal, solids, conduct electricity
mono
1

di
two

tri
three

tetra
four

penta
five
hexa
six

hepta
seven

octa
eight

nona
nine

deca
ten

dodeca
twelve

Expanded Octet Rule
When a central atom can have more than 8 shared electrons
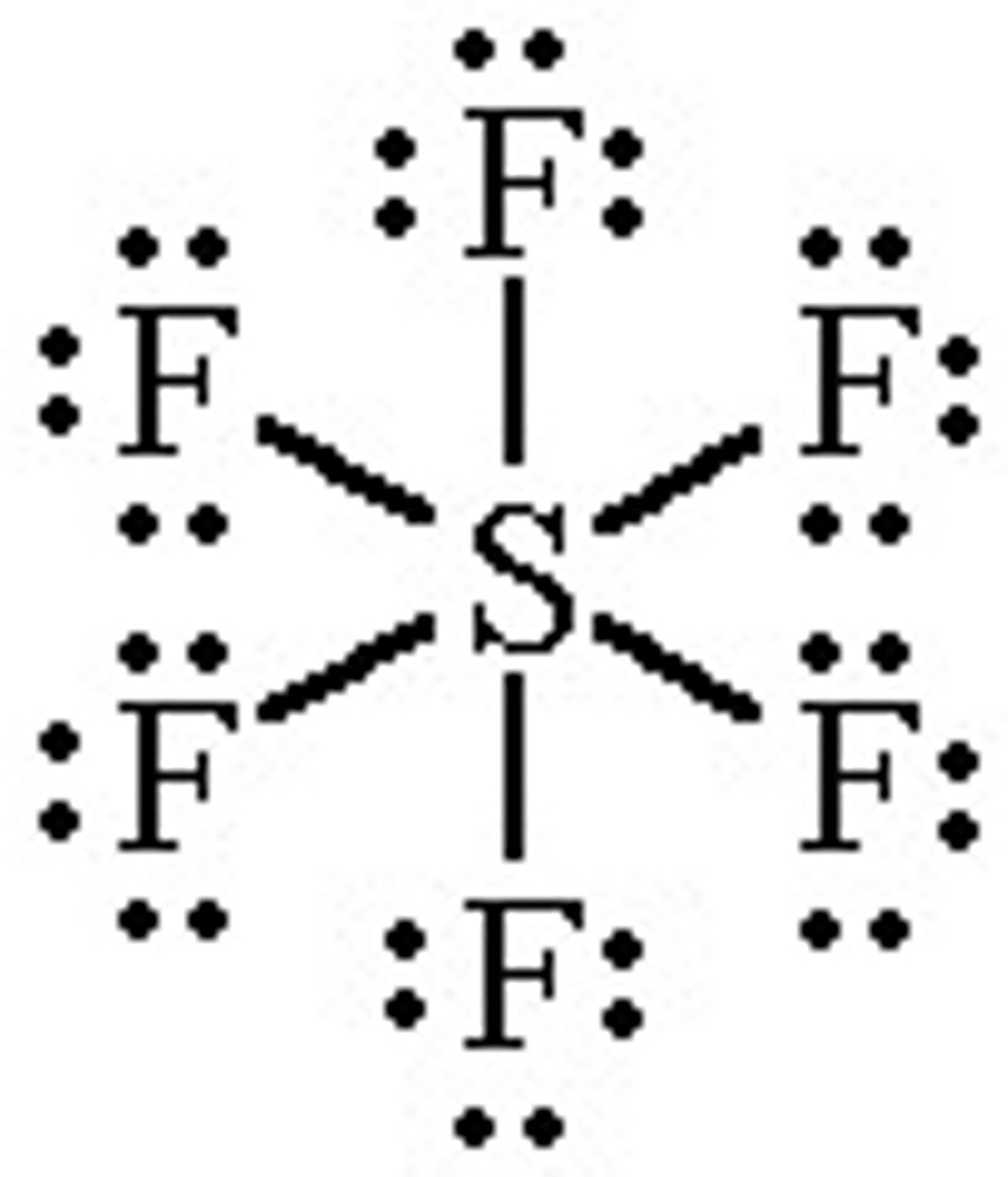
less than an octet of valence electrons
when an atom may have less than eight valence electrons (examples: hydrogen, boron, or a group 2 metal)