Determine mass of water in hydrated crystals
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
6 Terms
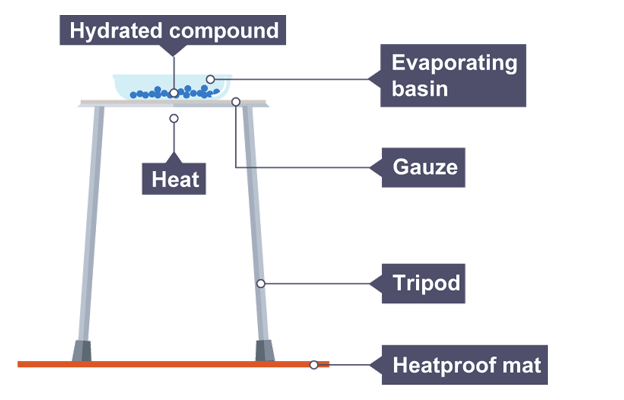
Apparatus
Hydrated iron(II) sulfate (FeSO4.xH2O)
Spatula
Bunsen burner, tripod and gauze
Heatproof mat
Evaporating basin
Tongs
Electronic balance
Stopclock
Method
Weigh evaporating basin and record mass value in results table
Keep evaporating basin on balance and add between hydrated iron(II) sulfate crystals
Record mass of the evaporating basin and crystals in results table
Place on gauze and heat gently for two minutes
Allow to cool and reweigh, recording masses in results table
Repeat until mass readings are the same (constant mass) so all water of crystallisation is removed
Observations
Steam given off from hydrated solid as it is heated
Can be colour change in hydrated solid
Crystals may break down into to a powder
Results
Use masses recorded in table to find mass of water of crystallisation
Error
not making sure flame is half open so it’s heated gently
Safety
Wear safety goggles as solid may spit when heated
Gentle heating to reduce risk of spitting
Heating could cause FeSO4 to decompose into iron(III) oxide and sulfur dioxide, so use well-ventilated lab
Allow hot apparatus to cool before touching it, to reduce the risk of burns
Iron(II) sulfate has a caution hazard symbol on the bottle
Wash hands if it touches your skin