General Chemistry 4: Compounds and stoichiometry
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
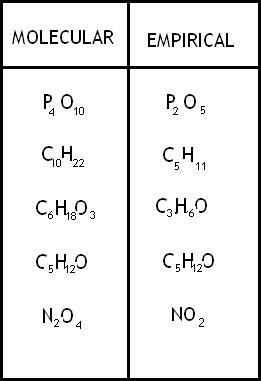
An [...] formula is the simplest whole-number ratio of atoms
empirical
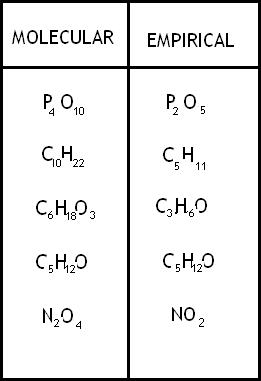
The [...] formula shows the exact number of atoms of each element
molecular
its is a multiple of the empirical formula
Two or more reactants forming one product
2H2 (g) + O2 (g) → 2H2O(g)
combination
Single reactant breaks down
2HgO(s) → 2Hg (l) + O2 (g)
decomposition
Involves a fuel, usually a hydrocarbon, and O2 (g)
Commonly forms CO2 and H2O
CH4 (g) + 2O2 (g) → CO2 (g) + H2O(g)
combustion
An atom/ion in a compound is replaced by
another atom/ion
Cu (s) + AgNO3 (aq) → Ag (s) + CuNO3 (aq)
single-displacement
Elements from two compounds swap places
CaCl2 (aq) + 2AgNO3 (aq) → Ca(NO3)2 (aq) + 2AgCl (s)
Double-displacement (metathesis)
A type of double-replacement reaction
Acid + base → salt + H2O
HCl (aq) + NaOH(aq) → NaCl (aq) + H2O(l)
Neutralization
For elements (usually metals) that can
form more than one positive ion, the
charge is indicated by a Roman numeral in
parentheses following the name of the
element
Fe2+ Iron(II)
Fe3+ Iron(III)
Cu+ Copper(I)
Cu2+ Copper(II)
Older method: –ous and –ic to the atoms
with lesser and greater charge,
respectively
Fe2+ Ferrous
Fe3+ Ferric
Cu+ Cuprous
Cu2+ Cupric
Monatomic anions drop the ending of the
name and add –ide
H- Hydride
F- Fluoride
O2- Oxide
S2- Sulfide
N3- Nitride
P3- Phosphide
Oxyanions = polyatomic anions that
contain oxygen.
MORE Oxygen = –ate
LESS Oxygen = –ite
NO3 - Nitrate
NO2 - Nitrite
SO42- Sulfate
SO3 2- Sulfite
In extended series of oxyanions, prefixes
are also used.
MORE Oxygen = Hyper- (per-)
LESS Oxygen = Hypo-
ClO- Hypochlorite
ClO2
- Chlorite
ClO3
- Chlorate
ClO4
- Perchlorate
Polyatomic anions that gain H+ to for
anions of lower charge add the word
Hydrogen or dihydrogen to the front.
HCO3
- Hydrogen carbonate or bicarbonate
HSO4
- Hydrogen sulfate or bisulfate
H2PO4
-
Dihydrogen phosphate
acid names
-ic: Have one MORE oxygen than -ous.
-ous: Has one FEWER oxygen than -ic.
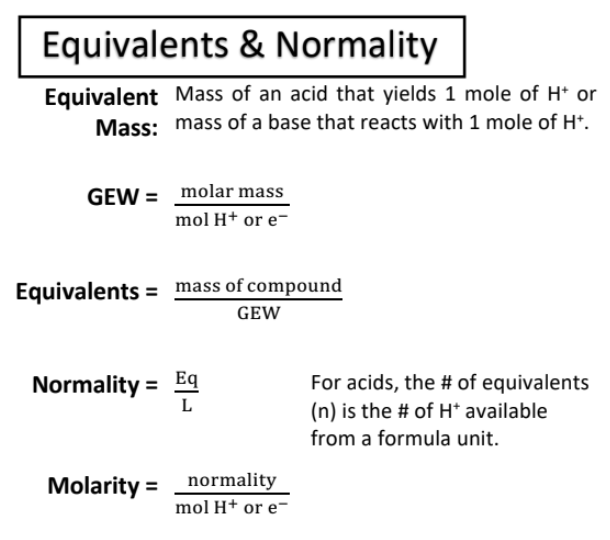
equivalents and normality