Physical/Inorganic Equations
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
Give 2 equations to show how we can use Magnesium to extract Titanium
TiO2 + 2Cl + 2C → TiCl4 + 2CO
TiCl4 + 2Mg → Ti + 2MgCl2
Explain how you can test for H2SO4
Add acidified BaCl2 to the solution of suspected H2SO4, A white solution of BaSO4 is produced.
Why is the test for H2SO4 unique?
No other ions other than Ba2+ give a positive result
Give the ionic equation for the test for H2SO4
Ba2+ + SO42- → BaSO4
Draw the table for displacement of group 7 ions
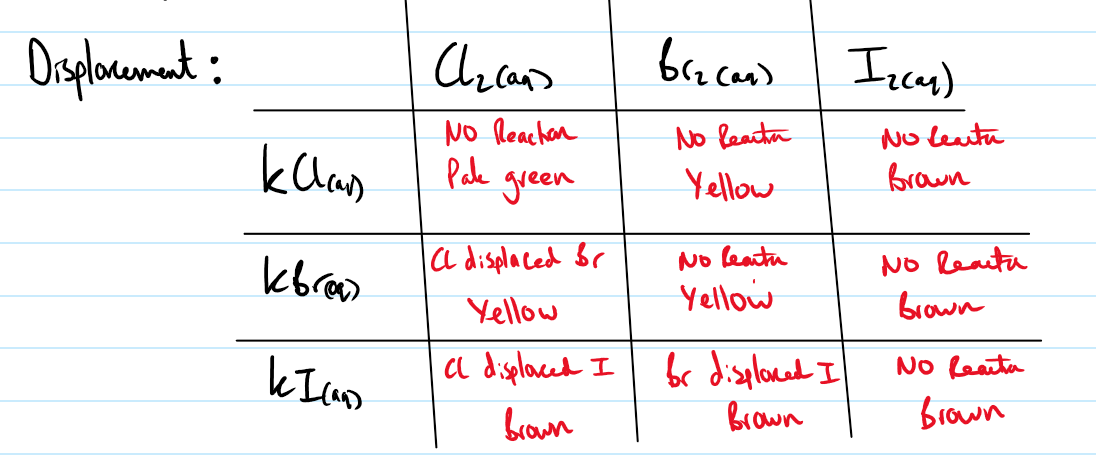
Give the equation and result of reacting silver nitrate with Cl- ions. Give a further test to distinguish between the other ions.
Ag+ + Cl- → AgCl (white precipitate)
+Dilute NH3 → Colourless
Give an equation to show the redissolving of the white precipitate AgCl.
AgCl + NH3 → Ag(NH3)+ + Cl-
Give the equation and result of reacting silver nitrate with Br- ions. Give a further test to distinguish between the other ions.
Ag+ + Br- → AgBr (Cream precipitate)
+Concentrated NH3 → Colourless
Give an equation to show the redissolving of the Cream Precipitate AgBr
AgBr + 2NH3 → [Ag(NH3)2]+ + Br-
Give the equation and result of reacting silver nitrate with I- ions. Give a further test to distinguish between the other ions.
Ag+ + I- → AgI (Yellow precipitate)
NO FURTHER TEST - AgI does not redissolve
Give the equation for the reaction between NaF and H2SO4
NaF + H2SO4 → NaHSO4 + HF
Give the equation for the reaction between NaCl and H2SO4
NaCl + H2SO4 → NaHSO4 + HCl
Give the first stage (acid-base) equation for the reaction between NaBr and H2SO4
NaBr + H2SO4 → NaHSO4 + HBr
Give the second stage (ionic) equation for the reaction between NaBr and H2SO4
2Br- + H2SO4 + 2H+ → Br2 + SO2 + 2H2O
Give the overall equation for the reaction between NaBr and H2SO4
2NaBr + 3H2SO4 → 2NaHSO4 + SO2 + Br2 + 2H2O
Name the products of the reaction between NaBr and H2SO4
NaHSO4
SO2
Br2
Give the observations of the reaction between NaBr and H2SO4
White steamy fumes of HBr
Orange fumes of Br
Colourless SO2
Give the first stage (acid-base) equation for the reaction between NaI and H2SO4
NaI + H2SO4 → NaHSO4 + HI
Give the second stage (ionic) equation for the reaction between NaI and H2SO4
2I- + H2SO4 + 2H+ → I2 + SO2 + 2H2O
Give the third stage (ionic) equation for the reaction between NaI and H2SO4
6I- + H2SO4 + 6H+ → 3I2 + S + 4H2O
Give the fourth stage (ionic) equation for the reaction between NaI and H2SO4
8I- + H2SO4 + 8H+ → 4I2 + H2S + 4H2O
Name the products of the reaction between NaI and H2SO4
HI
I2
SO2
S
H2S
Give the observations for the reaction between NaI and H2SO4
White steamy fumes of HI
Purple fumes of I
Colourless SO2
Yellow precipitate S
Colourless H2S (eggy smell)
Give a disproportionation reaction between chlorine and water
Cl2 + H2O ⇌ HClO + HCl
Give a reaction between chlorine and water in the presence of sunlight
2Cl2 + 2H2O → 4H+ + 4Cl- + O2
Give a reaction between chlorine and cold dilute NaOH
Cl2 + 2NaOH → NaCl + NaClO + H2O
Give the trends for the solubilities of Group 2 Hydroxides and Sulfates as you go down the group.
OH- : Compounds become more soluble as you go down the group
SO42-: Compounds become less soluble as you go down the group