133 lab_Activity 1: Interpreting Prescriptions + Unit of Measurements
1/171
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
172 Terms
Prescription
Regardless of the setting, prescriptions must be thoroughly evaluated for clarity, validity, and completeness by the pharmacist prior to dispensing.
is a written order and instruction of a validly registered physician, dentist, or veterinarian for the use of a specific drug product for a specific patient.
Medication orders
In hospital settings, prescriptions are called?
Medication order
the doctor’s order on the patient’s chart for the use of specific drugs
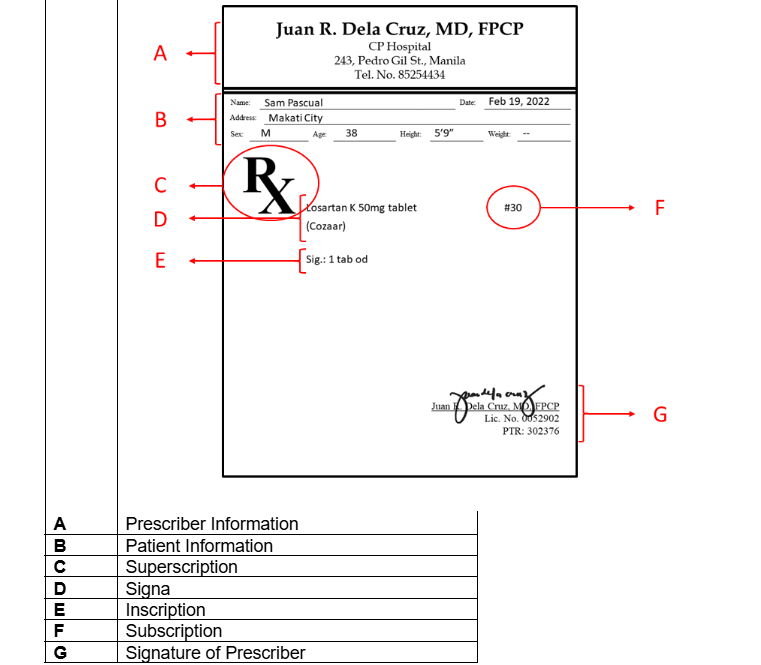
Superscription
Inscription
Subscription
Signa
Name and signature of prescriber
Parts of a prescription [5]
For drugs with a single active ingredient, the generic name of that active ingredient shall be used in prescribing. For drugs with two or more active ingredients, the generic name as determined by the Philippine FDA shall be used in prescribing.
The generic name must be written in full, but the salt or chemical form may be abbreviated.
The generic name of the drug ordered must be clearly written on the prescription immediately after the Rx symbol, or on the order chart.
Parts of a prescription
In addition to the generic name, a brand name may also be indicated
Violative Prescriptions
Erroneous Prescriptions
Impossible Prescriptions
Guidelines on what to do with violative, erroneous and impossible prescriptions
Electronic Prescriptions (E-prescriptions)
Mandatory Requirements of a Legal Prescription
a. If written on a prescription pad, the brand name enclosed in parentheses shall be written below the generic name.
b. If written on a patient chart, the brand name enclosed in parentheses shall be written after the generic name.
More than one drug product can be prescribed on one prescription form
What should be observed when indicating the brand name on the following:
a. If written a prescription pad
b. If written on a patient chart
A. Violative Prescription
Where generic name is not written
A. Violative Prescription
B. Erroneous Prescription
C. Impossible Prescription
A. Violative Prescription
Where the generic name is not legible and brand name is legibly written
A. Violative Prescription
B. Erroneous Prescription
C. Impossible Prescription
A. Violative Prescription
Where the brand name is indicated and instructions added (such as the phrase “no substitution”) which tend to abstract, hinder, or prevent proper generic dispensing
A. Violative Prescription
B. Erroneous Prescription
C. Impossible Prescription
B. Erroneous Prescription
Where the brand name precedes the generic name.
A. Violative Prescription
B. Erroneous Prescription
C. Impossible Prescription
B. Erroneous Prescription
Where the generic name is the one in parentheses.
A. Violative Prescription
B. Erroneous Prescription
C. Impossible Prescription
B. Erroneous Prescription
Where the brand name is not in parentheses.
A. Violative Prescription
B. Erroneous Prescription
C. Impossible Prescription
C. Impossible Prescription
When only the generic name does not correspond to the brand name.
A. Violative Prescription
B. Erroneous Prescription
C. Impossible Prescription
C. Impossible Prescription
When the generic name does not correspond to the brand name
A. Violative Prescription
B. Erroneous Prescription
C. Impossible Prescription
C. Impossible Prescription
. When both the generic name and the brand name are not legible
A. Violative Prescription
B. Erroneous Prescription
C. Impossible Prescription
A. Violative Prescription, C. Impossible Prescription
a. Violative and impossible prescriptions as defined by AO 62 (Generic Prescribing) shall not be filled. The pharmacist shall advise the prescriber of the problem and/or instruct the customer to get the proper prescription. These violative and wrong prescriptions shall be kept and reported by the pharmacist or other interested parties to the nearest DOH office for appropriate action.
b. Erroneous prescriptions shall be filled, but they shall also be kept and reported to the nearest DOH office for appropriate action
Guidelines on what to do with violative, erroneous and impossible prescriptions:
What shall not be filled by a pharmacist?
A. Violative Prescription
B. Erroneous Prescription
C. Impossible Prescription
one (1) week
Any e-prescription containing antibiotic, anti-infectives and antiviral preparations shall only be valid for [BLANK] after its issuance
[Term]
a. FDA Circular No. 2020-007 & 2020-037 were released in lieu of the COVID-19 pandemic to ensure continuous access of individuals vulnerable to COVID-19 to their prescribed medicines during the strict quarantine and lockdown period. These provided guidelines on the issuance of e-prescriptions by licensed physicians and dispensing of medicines based on valid e-prescriptions.
b. the optical electronic data (captured image in pdf, jpeg or other photo file format) issued by or made by a licensed physician which is generated, sent, received or stored through email and messaging applications. The said electronic document shall contain the medical prescription needed by the individuals vulnerable to COVID-19 and the electronic signature of the licensed physician.
Electronic Prescriptions
c. Dispensing guidelines for written prescriptions apply to dispensing of e-prescriptions.
d. Any e-prescription containing antibiotic, anti-infectives and antiviral preparations shall only be valid for one (1) week after its issuance
Dispensing
In the Philippines, pharmacists are required to perform generic dispensing by informing the patient of all available drug products generically equivalent to the one prescribed with their corresponding prices.
refers to all activities related with the sale or transfer of pharmaceutical products, with or without prescription, by the pharmacist
A prescription may be complete filled when the pharmacist provides the complete number of medicines indicated in the prescription. After which, the pharmacist keeps the prescription in the pharmacy for filing.
As for partial filling, the pharmacist is required to write the following information on the prescription before returning it to the client:
• The date of partial filling
• Quantity served and balance of the prescription unserved
• Name and address of drugstore
Complete filling vs Partial filling
The date of partial filling
Quantity served and balance of the prescription unserved
Name and address of drugstore
As for partial filling, the pharmacist is required to write the following information on the prescription before returning it to the client:
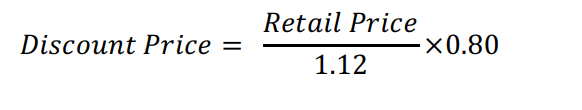
Senior citizens and PWD discounts should be acknowledged and applied when dispensing prescription drugs. This includes a grant of 20% discount and exemption from value-added tax (VAT) upon presenting the required documents to be computed as follows: what formula for discount price ?
The Mexico City and Kuala Lumpur Principles are the primary Codes of Ethics in the Biopharmaceutical Sector and Medical Device Sector, respectively. Both Codes of Ethics have been adopted by the Philippine FDA5 to ensure that information dissemination, advertisements, promotion, sponsorship, and other marketing activities and instruments about prescription pharmaceutical products and medical devices safeguard patient rights and welfare.
The Codes provide a framework on the ethical relationship between healthcare professionals and the pharmaceutical industry which ensures that professional practice is based on truthful, accurate, and updated scientific evidence without any form of bias towards any pharmaceutical product.
Further, it also provides guidelines on contents of promotional materials to adequately demonstrate the balance between risks and benefits of pharmaceutical products, comply with existing FDA and other pertinent regulations, and to ensure that claims are substantiated with up-to-date scientific evidence. Other pertinent regulations to ensure the ethical promotion of pharmaceutical products such as sponsorships, use of physician samples and discount cards are found in the Philippine FDA adopted guidelines through DOH AO 2015-0053.
[Promotion and Marketing of Prescription Pharmaceutical Products and Medical Devices]
are the primary Codes of Ethics in the Biopharmaceutical Sector and Medical Device Sector,
2.54 cm
1 inch = ? cm
39.37 in
1 m =? in
29.57 mL
1 fl oz = ? mL
473 mL
1 pint (16 fl oz) = ? mL
946 mL
1 quart (32 fl. oz.) = ___ mL
3785 mL
1 gallon, US (128 fl. oz.) =___ mL
4545 mL
1 gallon (UK) = __ mL
454 g
1 pound (lb) in grams?
2.2 pounds (lb)
1 kilogram (kg) in pounds (lb)?
before meals
a.c. (ante cibos)
at pleasure, freely
ad lib. (ad libitum)
administer
admin
A.M.
morning
water
aq.
around the clock
ATC
twice a day
b.i.d.
with
c
day
d
dilute
dil
and
et
hour
h. or hr. (hora)
at bedtime
h.s. (hora somni)
i.c. (inter cibos)
between meals
minute
min.
morning and night
m&n
nausea and vomiting
N&V
night
noct. (nocte)
nothing by mouth
NPO (non per os)
after meals
p.c. (post cibos)
afternoon; evening
P.M.
by mouth; orally
p.o. (per os)
as needed
p.r.n. (pro re nata)
every
q (quaque)
every morning
qAM
every X hours
qXh
four times a day
q.i.d. (quarter in die)
repeat
rep.
without
s (sine)
once a day
s.i.d. (semel in die)
if there is need; as needed
s.o.s
immediately
stat. (Statim)
3 times a day
t.i.d (ter in die)
times
X
as directed
ut dict. (ut dictum)
week
wk.
of each
aa. (or ana)
up to; to make
ad
dispense
disp.
div.
divide
give of such doses
d.t.d. (dentur tales doses)
make
ft (fiat)
mix
M. (mice)
number
No.
do not repeat
non rep. or NR
a sufficient quantity
q.s.
a sufficient quantity to make
q.s. ad
write (directions on label)
Sig. (Signa)
body surface area
BSA
cubic centimeter
cm3
fluid
f or fl
fluid dram (~ 5 mL)
fl℥
half-fluidounce (~15 mL)
fl℥ss or f℥ss
gram
g
gallon
gal
drop
gtt (gutta)
pound
lb (libra)
kilogram
kg
square meter
m2 or M2
microgram
mcg / ug
milliequivalent
mEq
mg/kg
milligrams (of drug) per kilogram (of body weight)
milligrams (of drug) per square meter (of body surface area)
mg/m2
milliliter
mL
pint
pt.
quart
qt
one half
ss (semissem)
tablespoonful
tbsp