Biological molecules
1/126
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
127 Terms
What are carbohydrates made of?
C, H, O
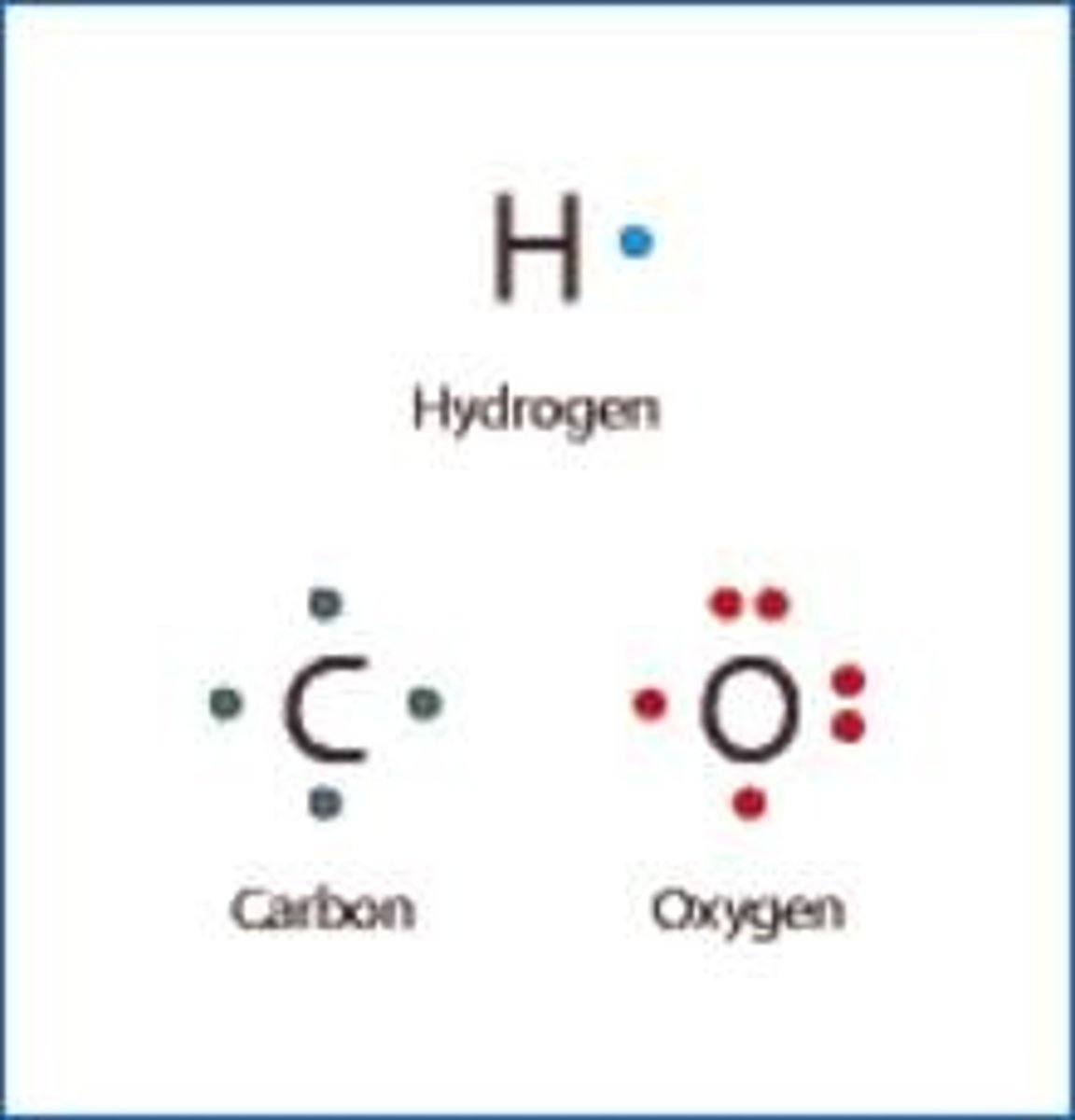
What are lipids made of?
C, H, O
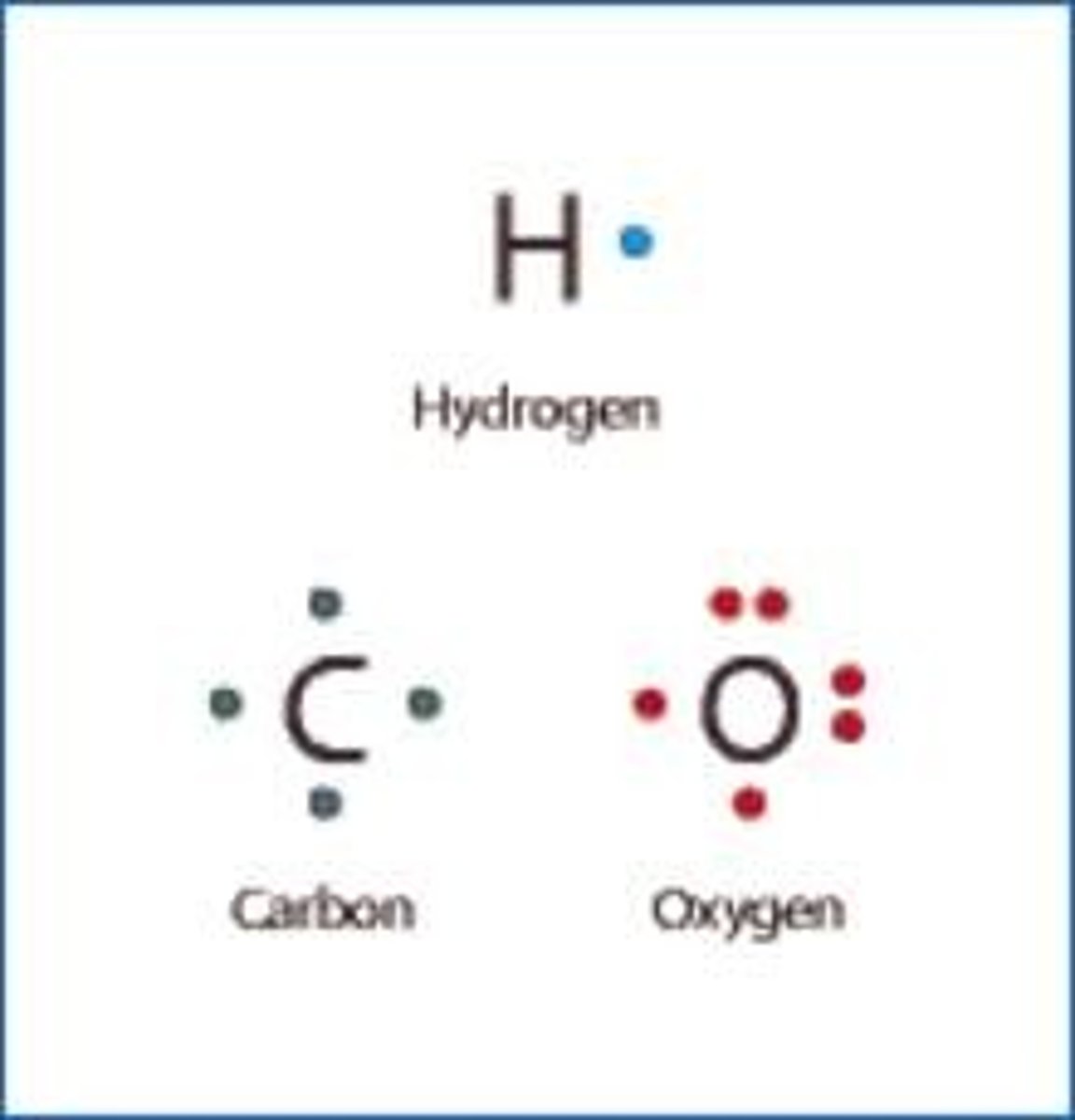
What are proteins made of?
C, H O, N, S
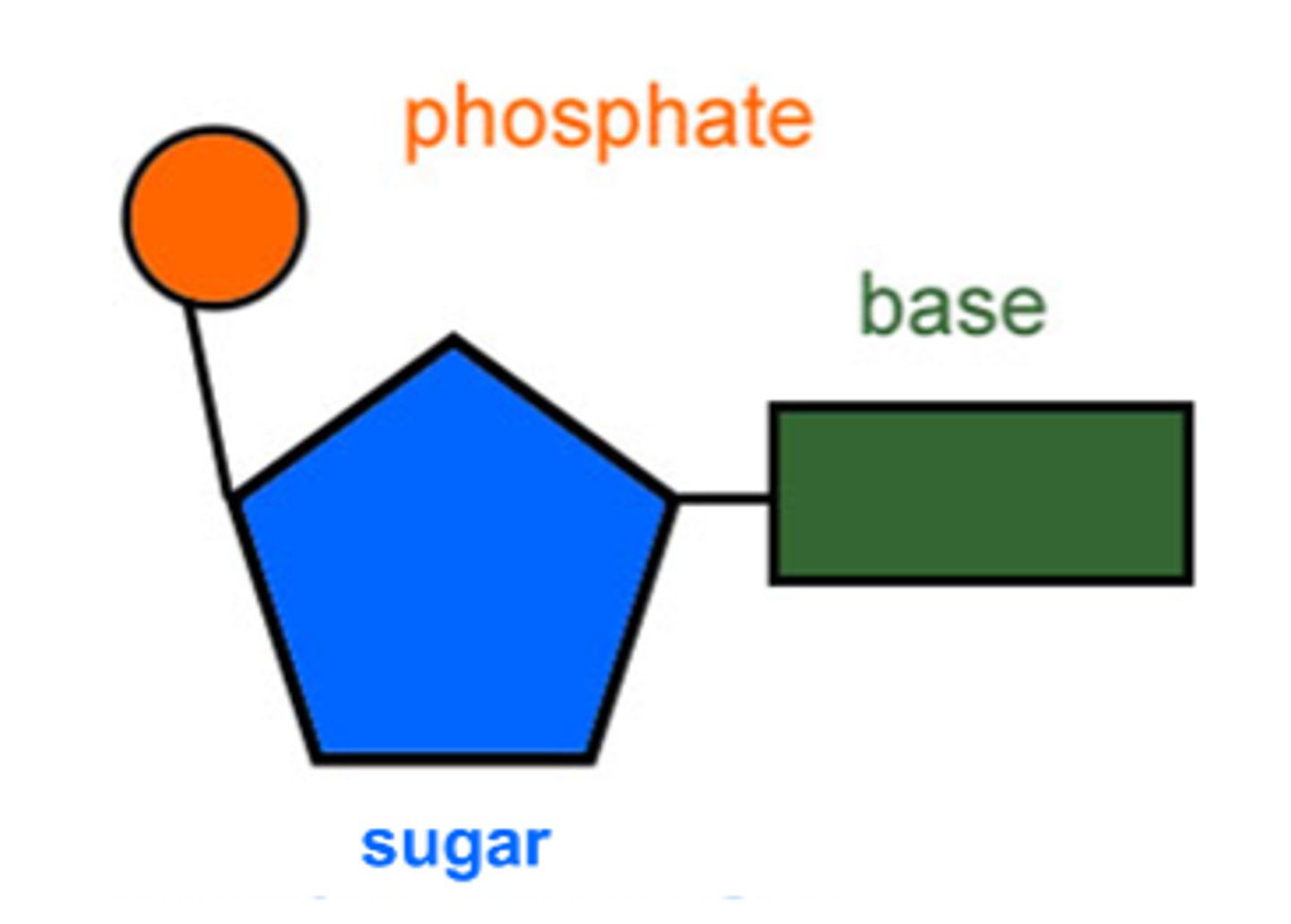
What are nucleic acids (DNA/RNA) made of?
C, H, O, N, P
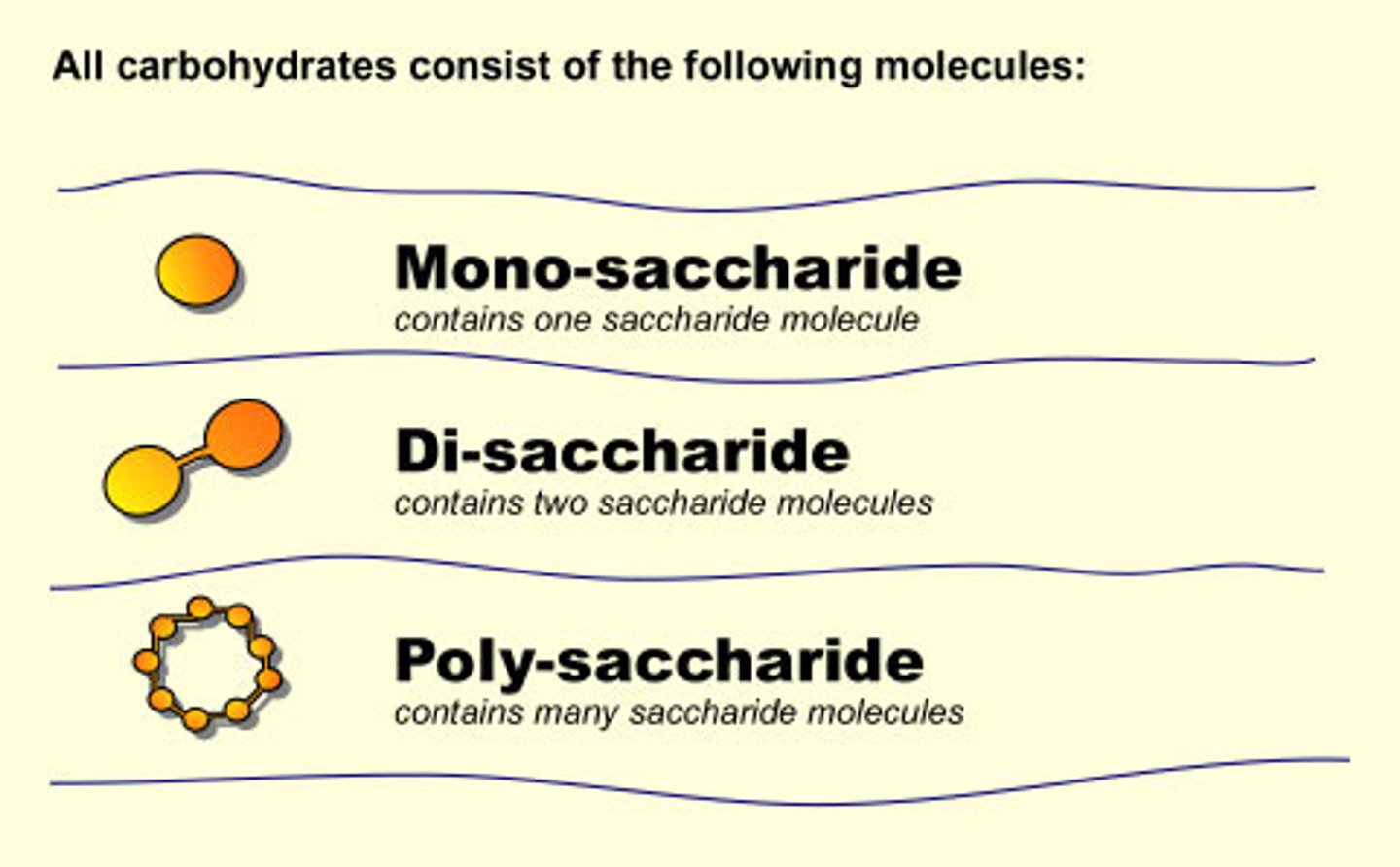
Types of Carbohydrates
Monosaccharides, Disaccharides, Polysaccharides
Properties of Glucose
- Hexose monosaccharide (6 carbon atoms)
- Highly soluble
- Major energy source
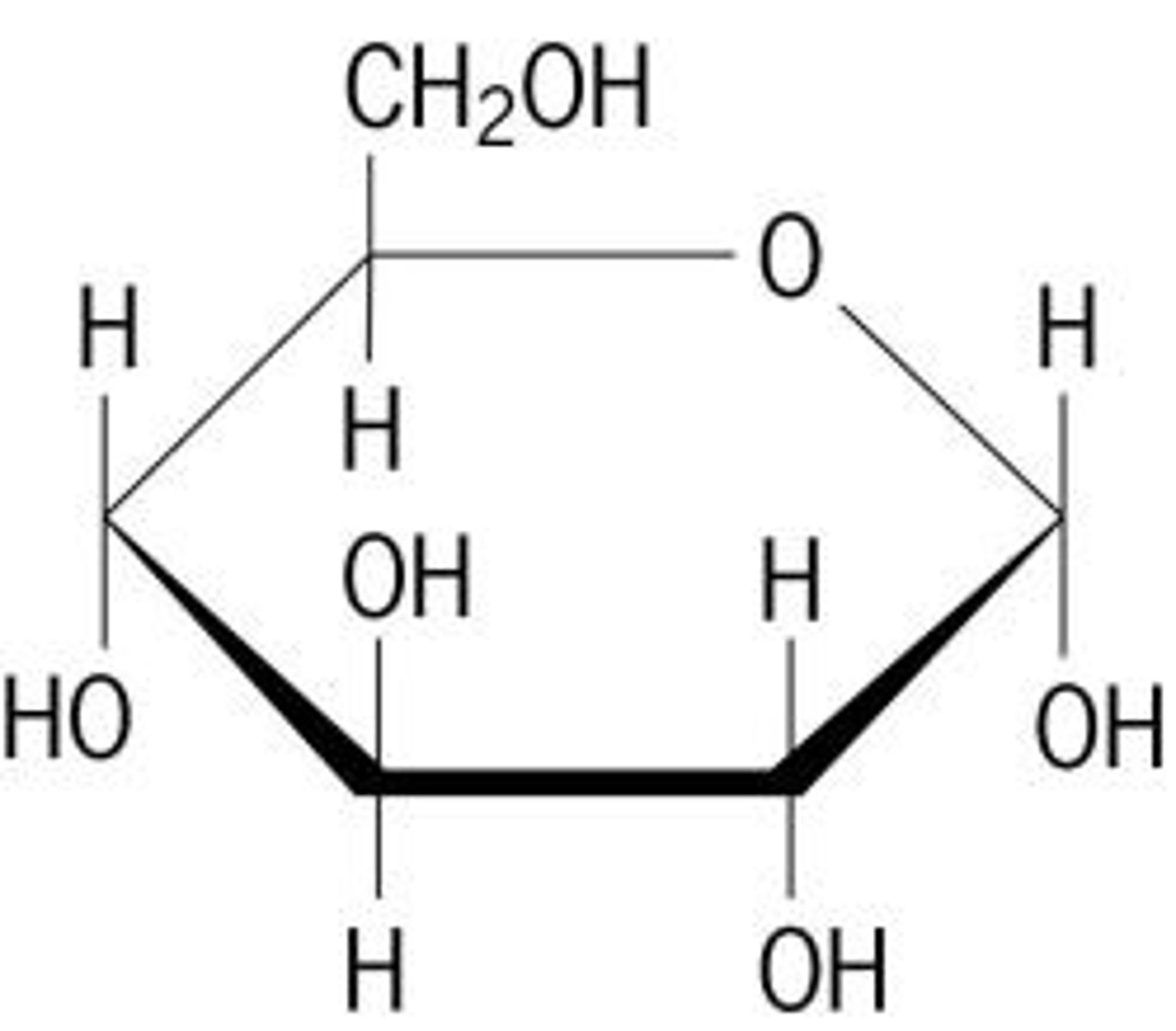
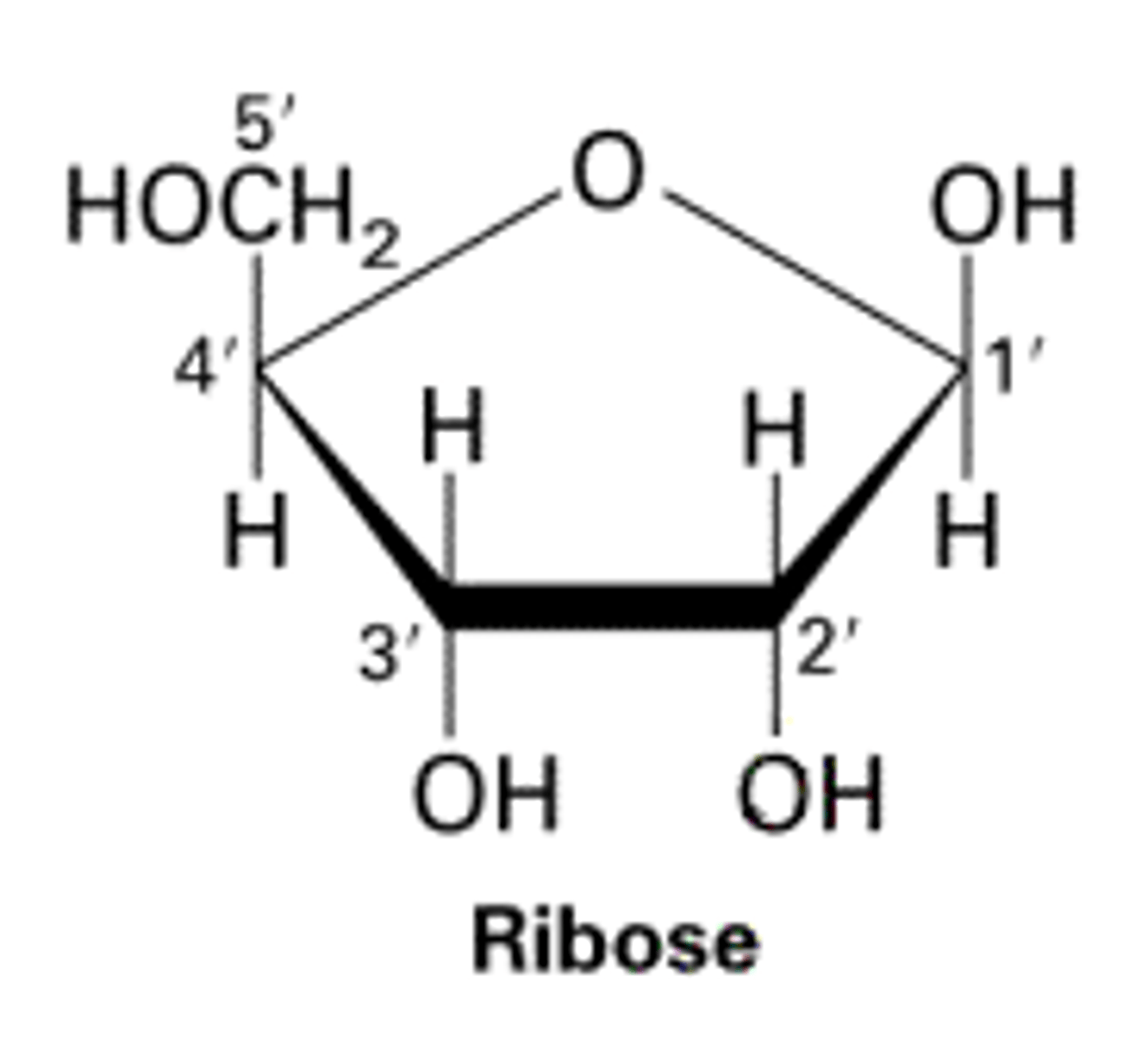
What are pentose monosaccharides?
Sugars with 5 carbon atoms (eg; Ribose, Deoxyribose)
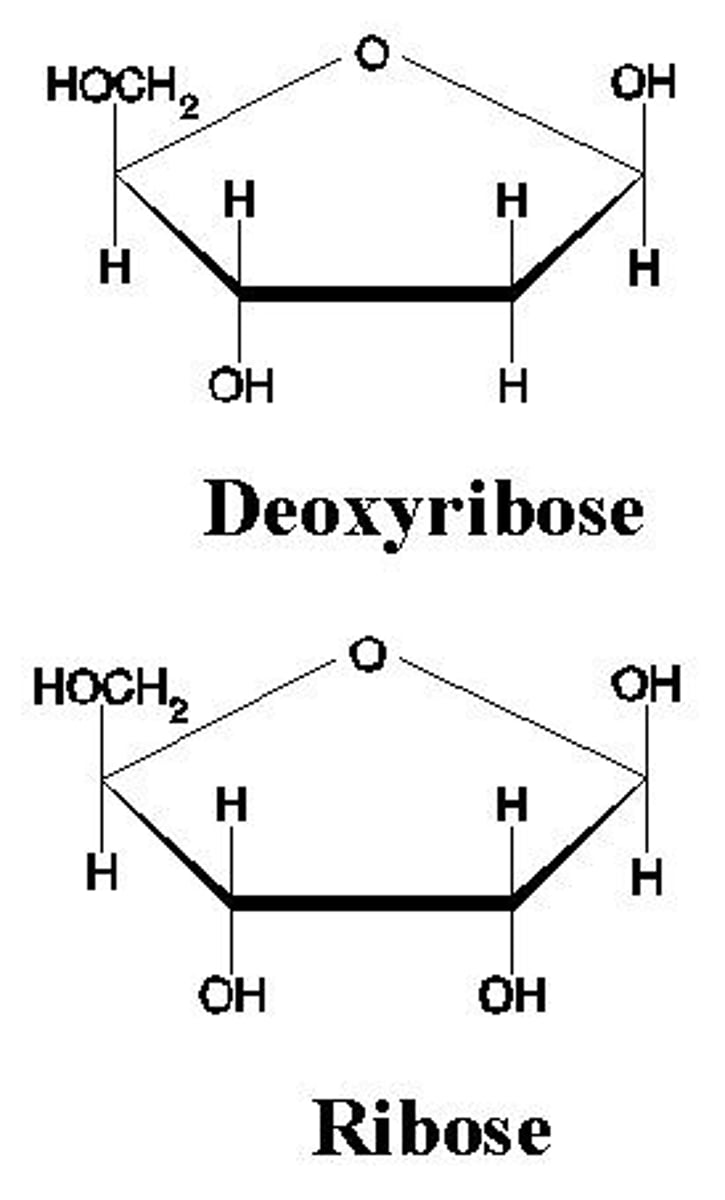
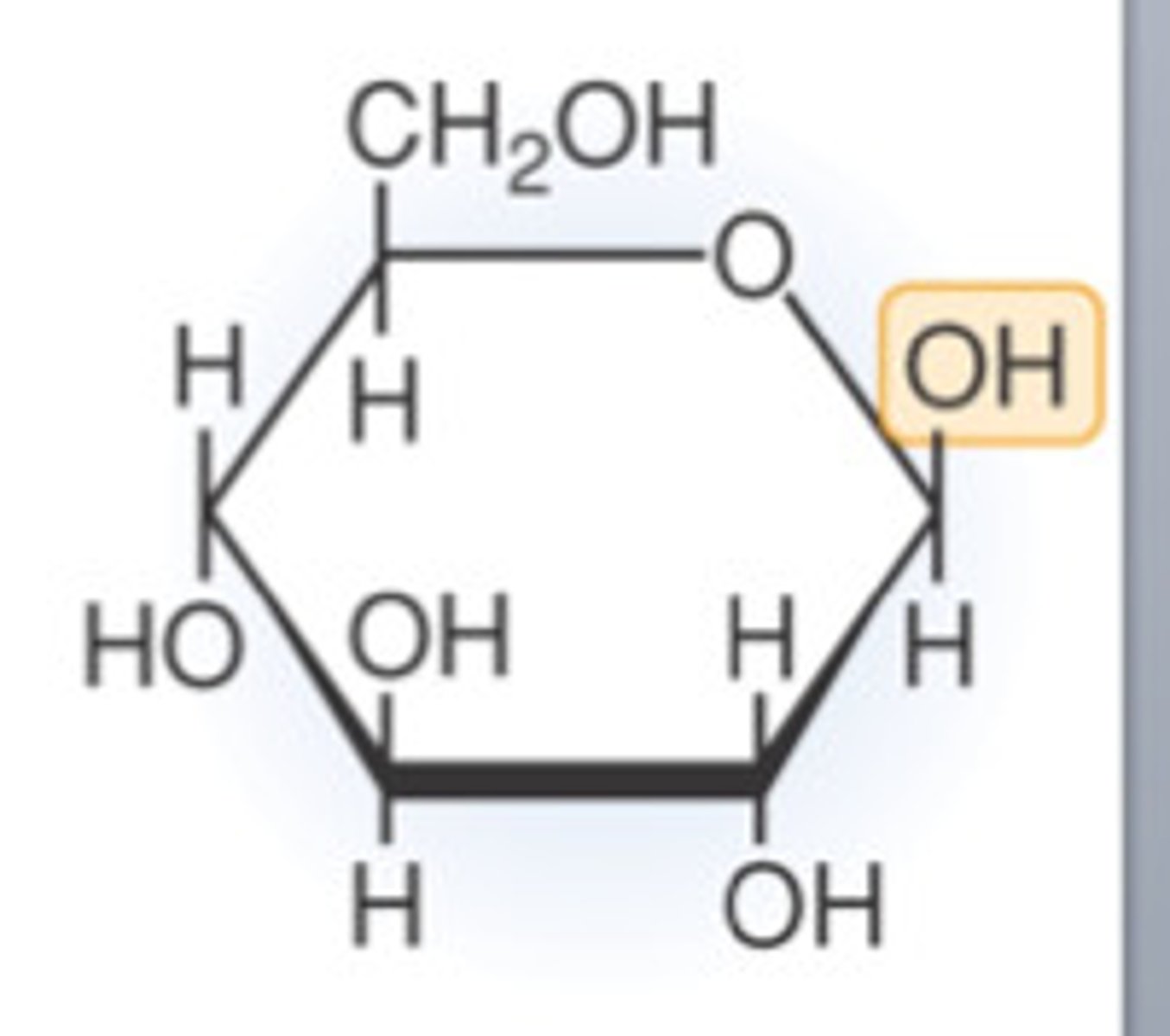
Structure of alpha glucose
OH down
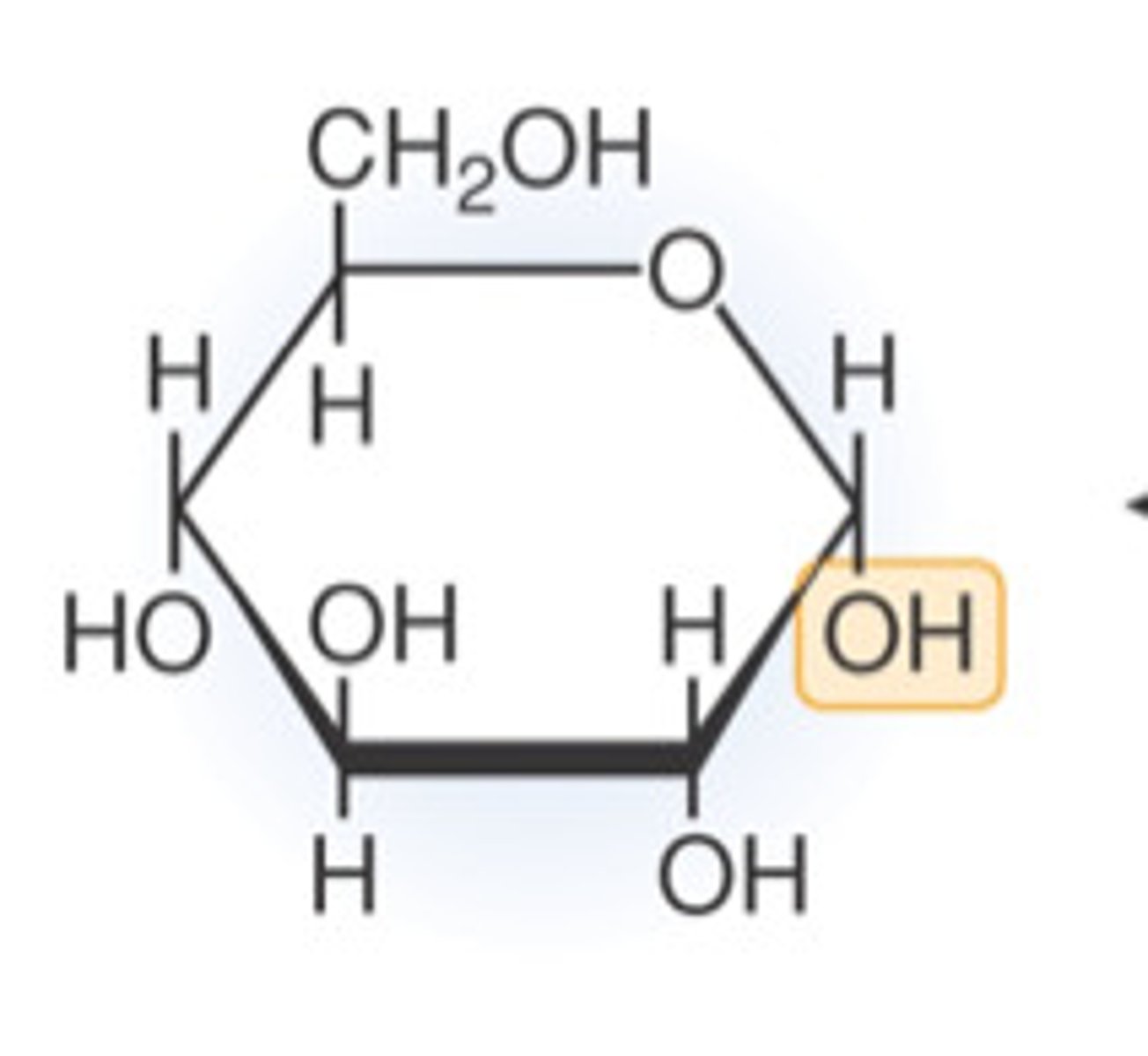
Structure of beta glucose
OH up
Structure of ribose
Disaccharaide
2 monosaccharides bonded by a glycosidic bond
How are disaccharides and polysaccharides formed?
Condensation reaction releasing a water molecule
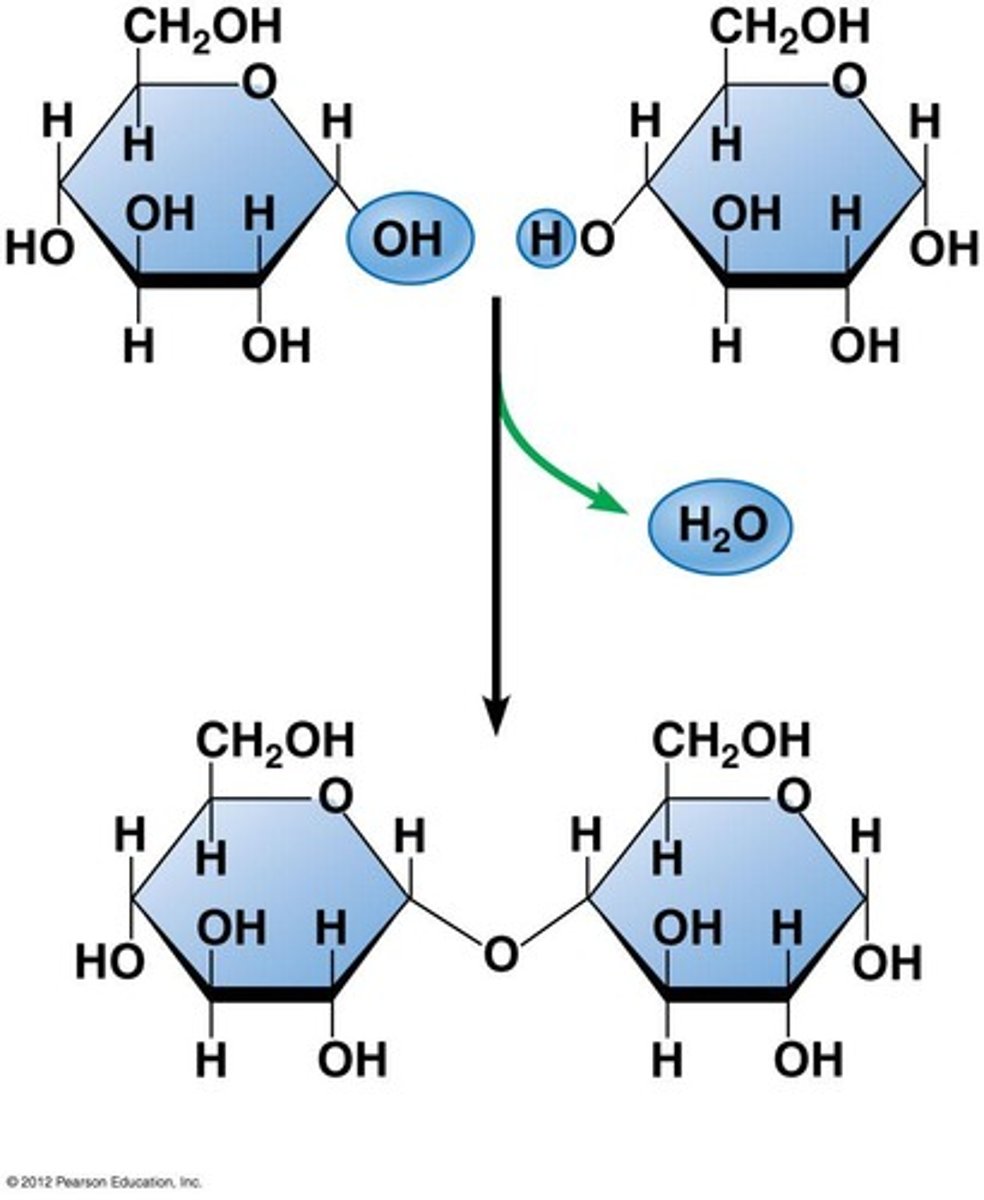
How are disaccharides broken down?
By hydrolysis using a water molecule
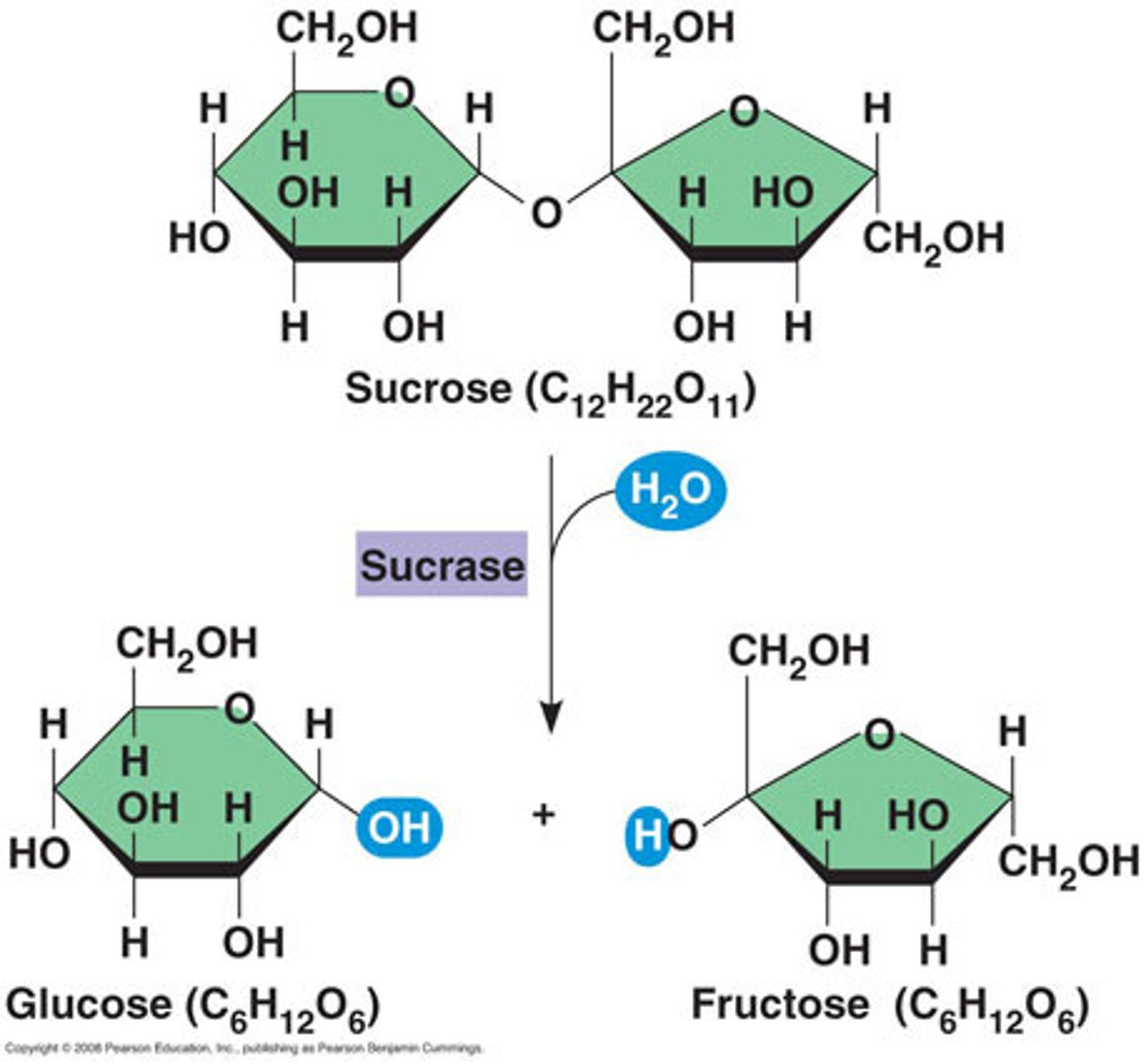
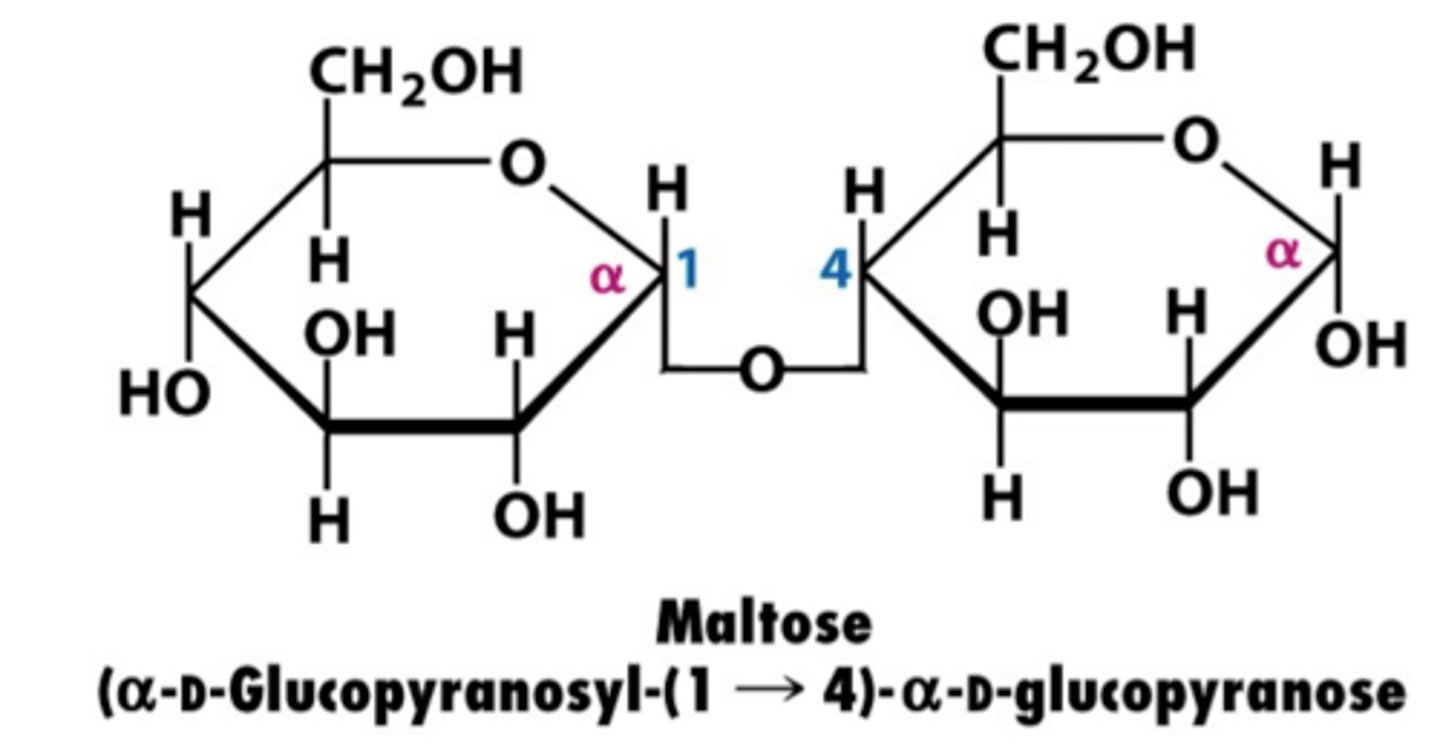
What is maltose made of?
- 2 Alpha Glucose molecules
- Alpha 1-4 glycosidic bond
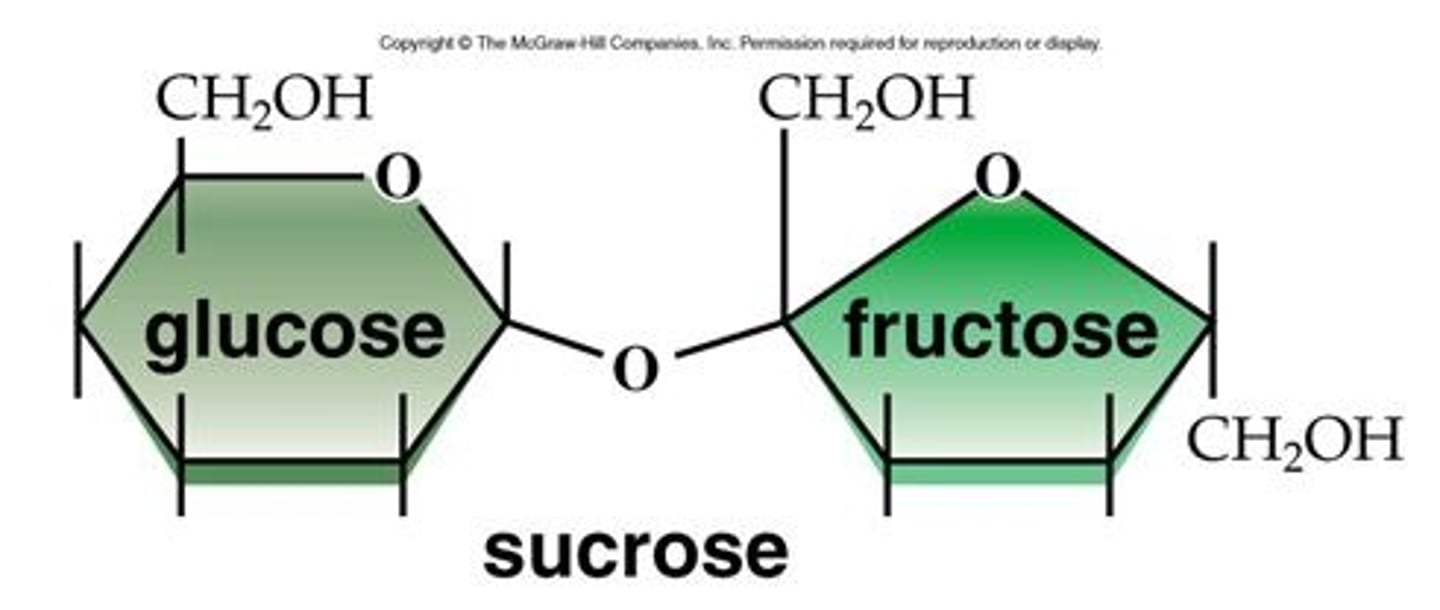
What is sucrose made of?
- Alpha Glucose + Fructose
- Alpha 1-5 glycosidic bond
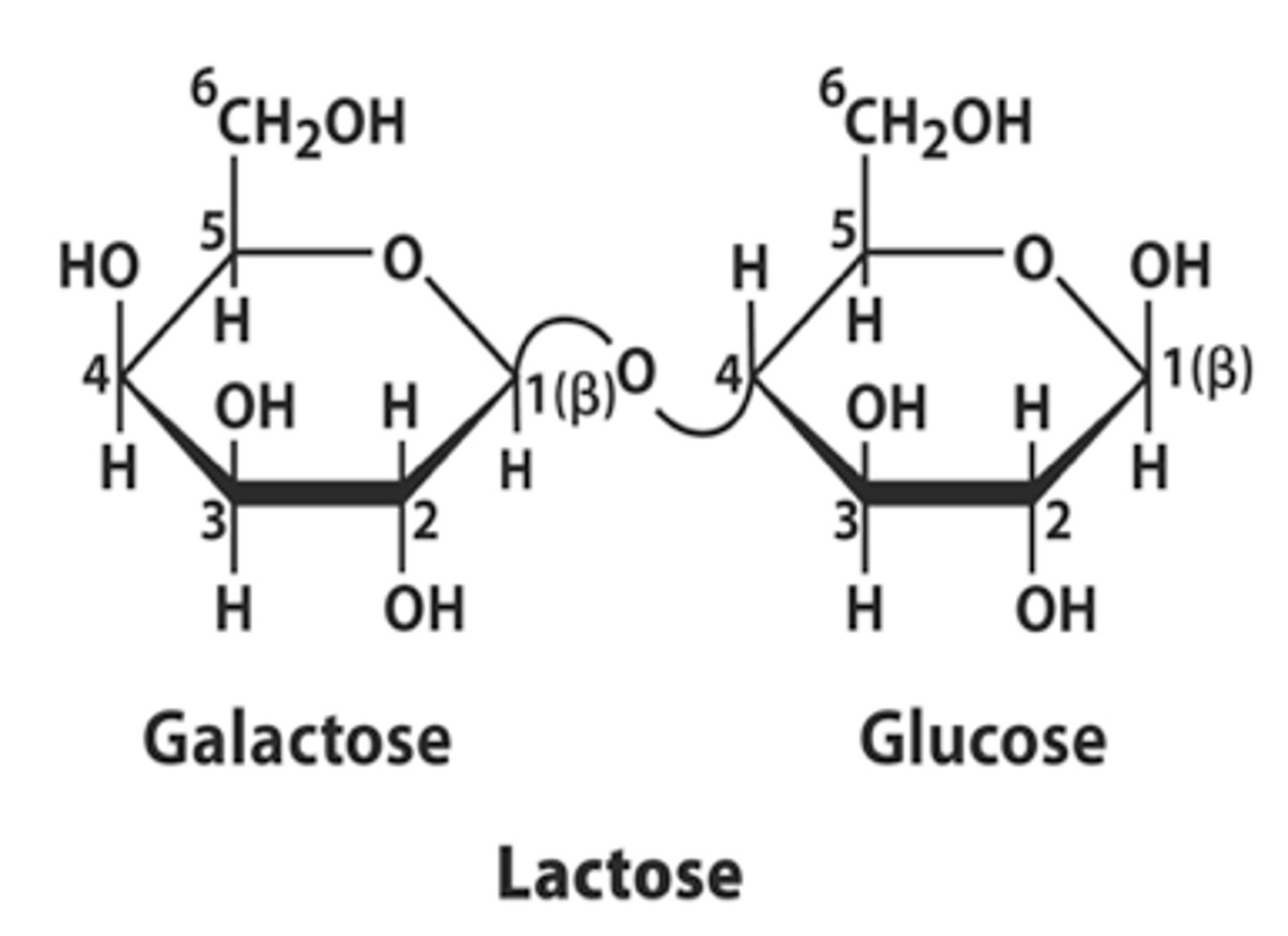
What is lactose made of?
- Alpha Glucose + Galactose
- Alpha 1-4 glycosidic bond
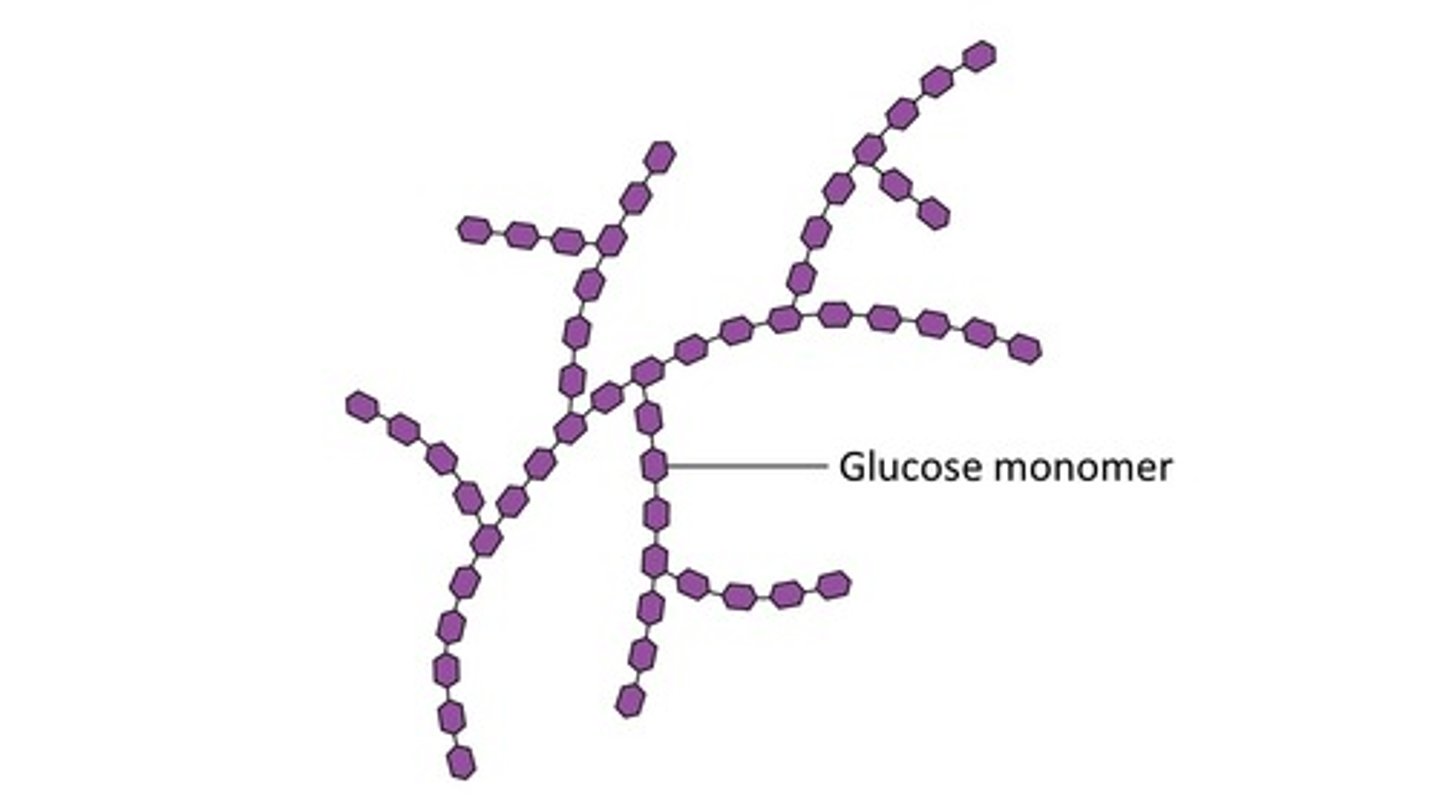
Structure of Glycogen
- Made of Alpha Glucose
- 1,4 and 1,6 glycosidic bonds
- Highly Branched structure
- Used for energy storage
Why is it good that Glycogen molecules are branched?
Increases SA so glucose can be rapidly hydrolysed and released

Structure of Cellulose
- Made of Beta Glucose
- Beta 1,4 glycosidic bonds
- Strong due to hydrogen bonds between microfibrils (long cellulose chains)
- Used for cell walls
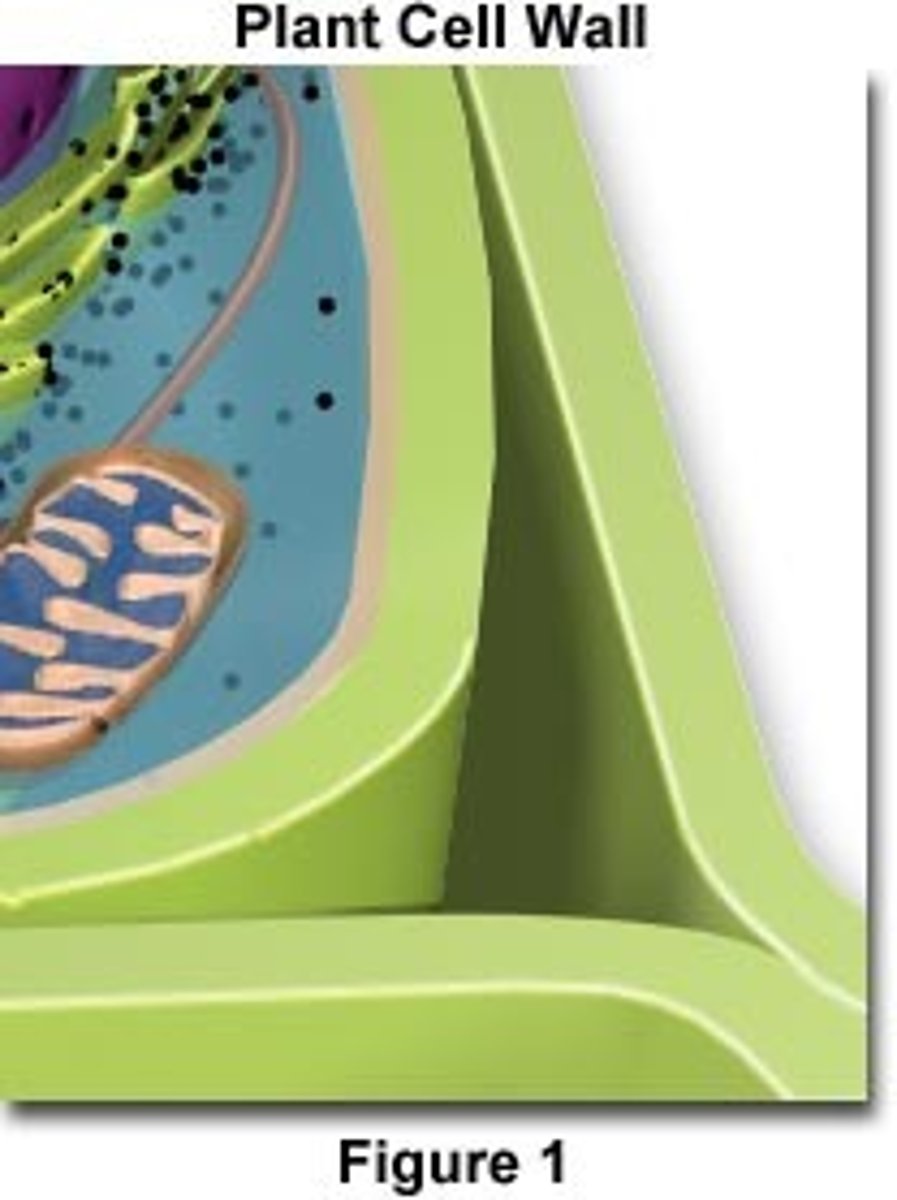
Properties of Cellulose
- Insoluble
- Unreactive
- Flexible
- Form hydrogen bonds with neighbouring chains
Structure of Starch
- Made of Alpha Glucose
- Consists of Amylose and Amylopectin polymers
- Used for energy storage
- Insoluble in water (doesn't affect cell's water potential)
- Helical shape (compact storage)
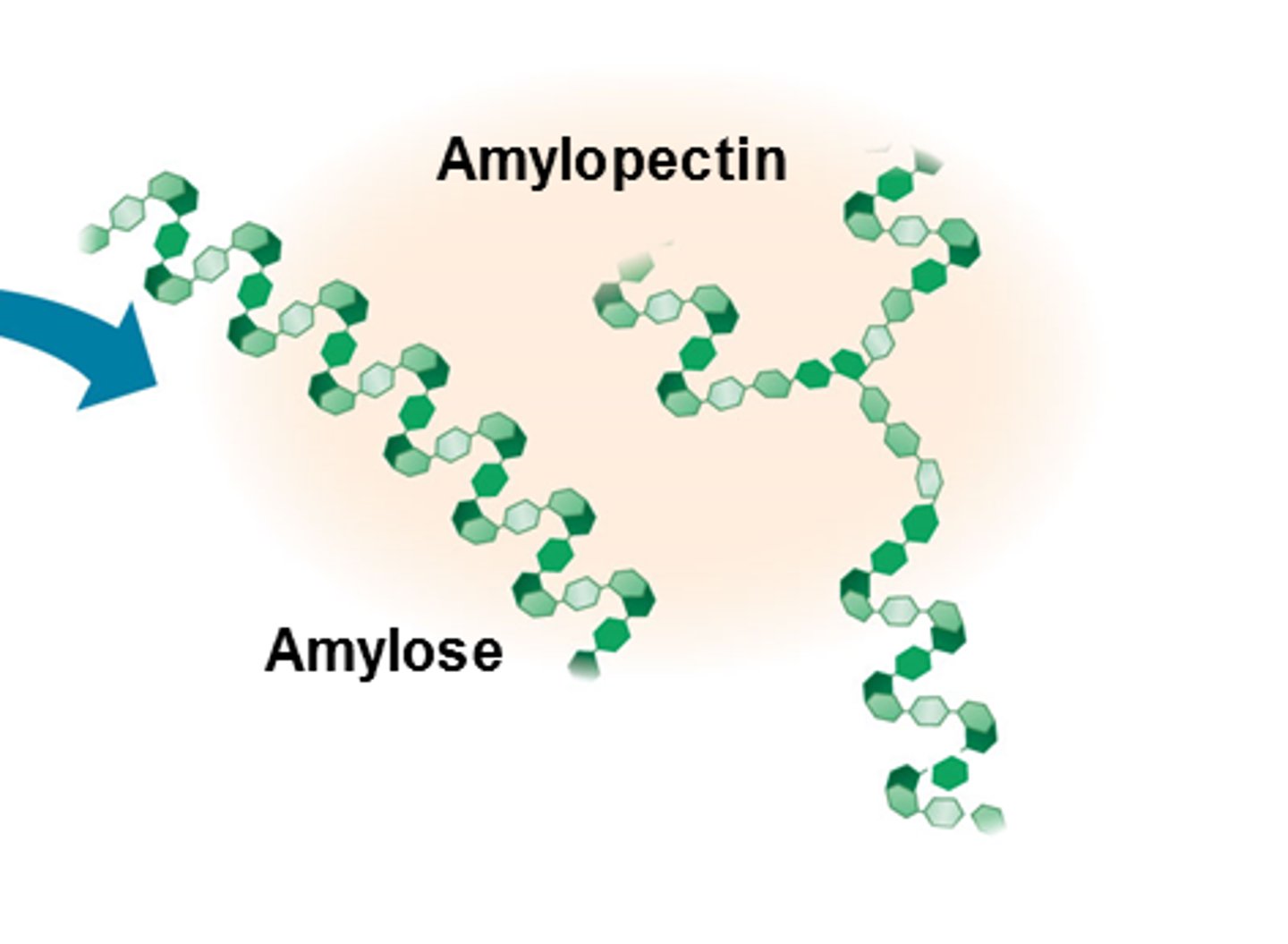
What is amylose?
- Unbranched chain of alpha glucose
- 1,4 glycosidic bonds
- Compact helical chain (stores lots of energy)
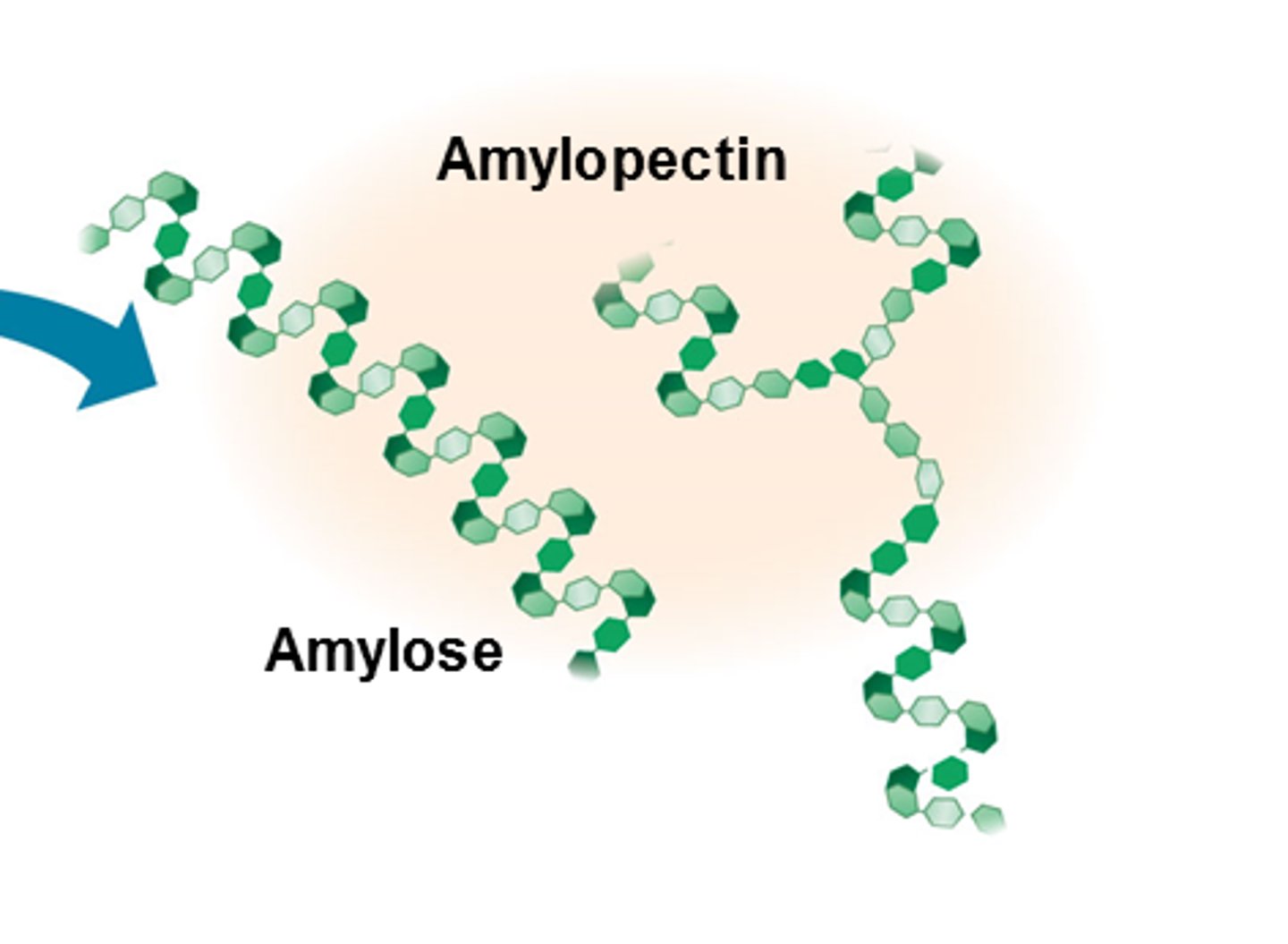
What is amylopectin?
- Branched chain of alpha glucose
- 1,4 and 1,6 glycosidic bonds
- Branches mean energy is quickly hydrolysed into glucose
Functions of Water
- Solvent
- Transport medium
- Coolant
- Habitat
Why is water a good solvent?
Molecules are polar so enables water can bind to solute molecules
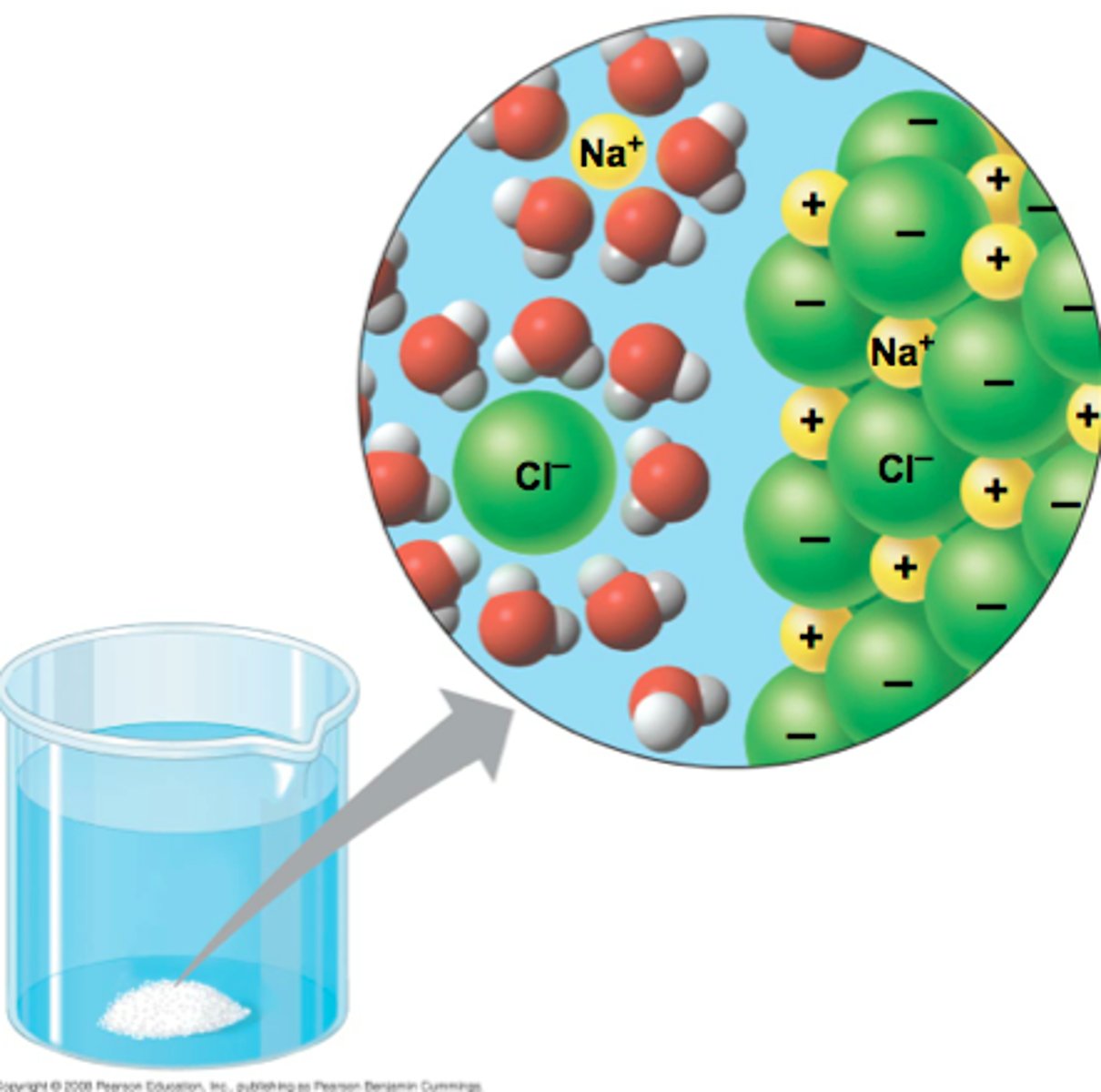
Why is water a good transport medium?
Cohesion due to hydrogen bonds between molecules so water can flow (eg xylem)
Properties of Water
- Ice is less dense than water
- High specific heat capacity
- High latent heat of vaporisation
- Cohesion produces surface tension
- Adhesion means water is attracted to surfaces
- Reactant
Why is water a good habitat?
- Ice is less dense than water
- Ice provides habitat and insulates water below
- Animals can move and oxygen can circulate

Why is water a good coolant?
- High specific heat capacity so internal temperature of organisms remains constant (enzymes aren't affected)
- High latent heat of vaporisation
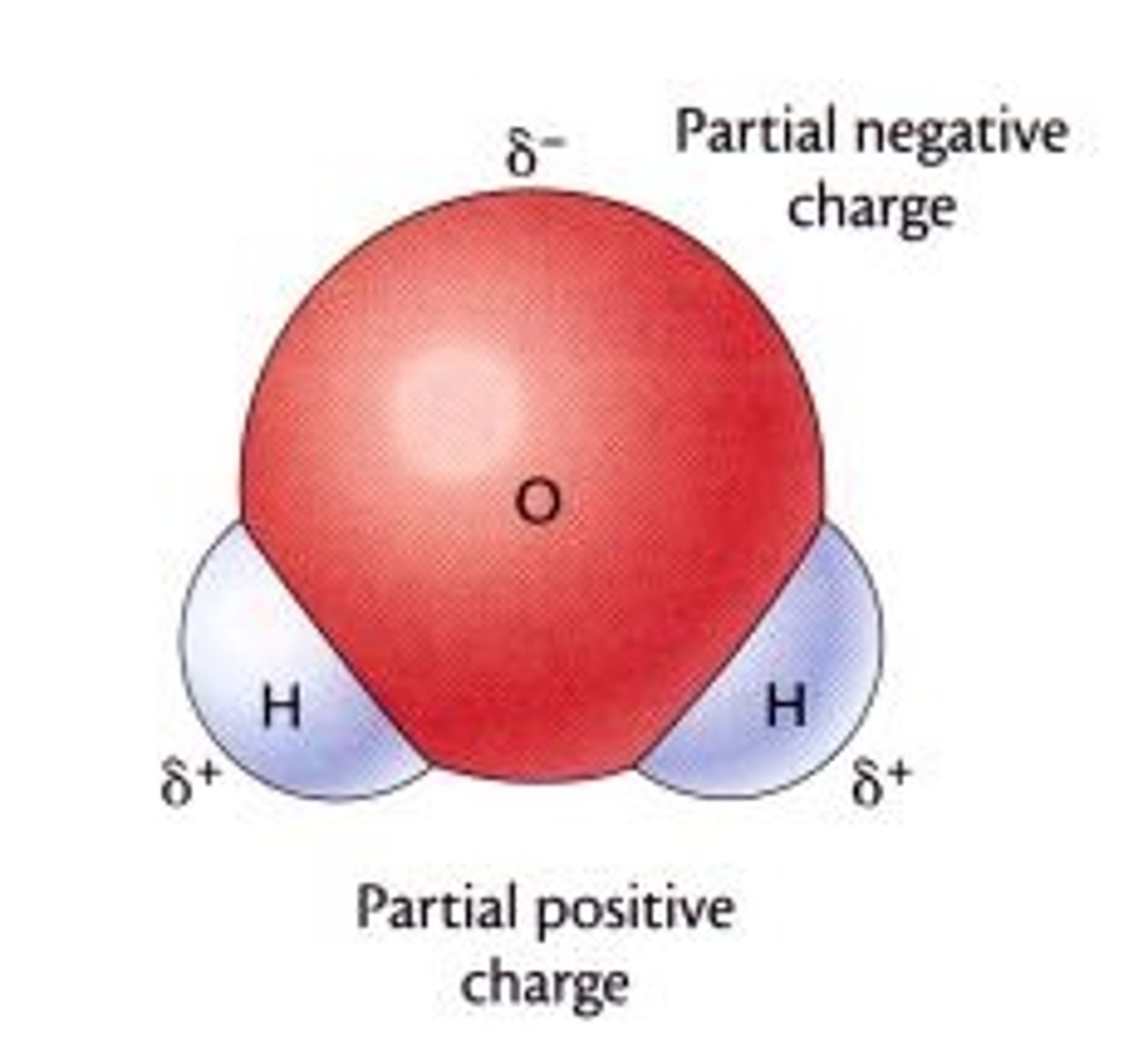
Why is water polar?
Oxygen is delta negative (δ–) whereas hydrogen is delta positive (δ+)
- Oxygen atom pulls the shared electrons towards it, so water is slightly negative at the oxygen and slightly positive at the hydrogen ends meaning they can form hydrogen bonds with each other
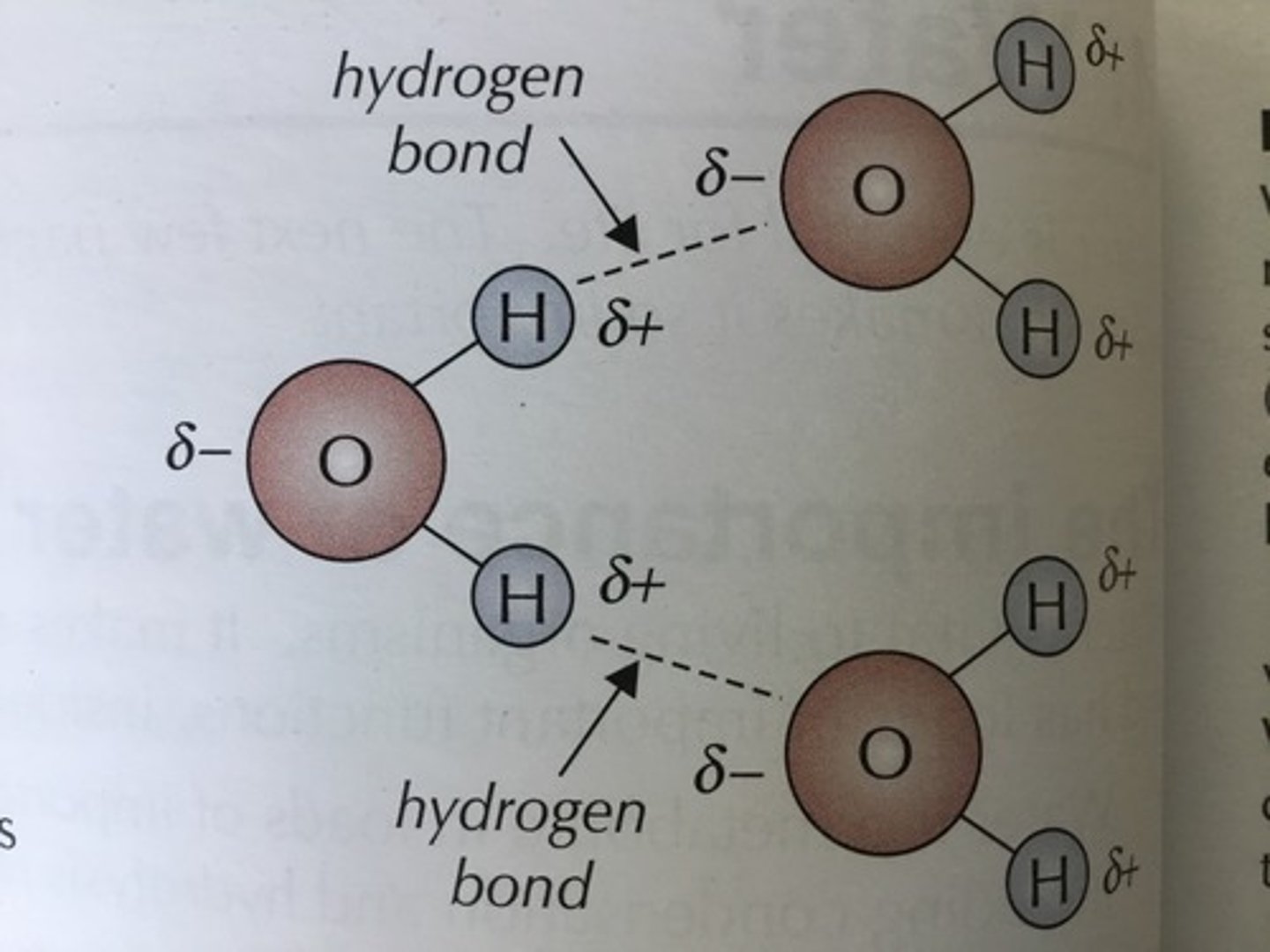
How do water molecules bond together?
Hydrogen bonds due to water's polarity
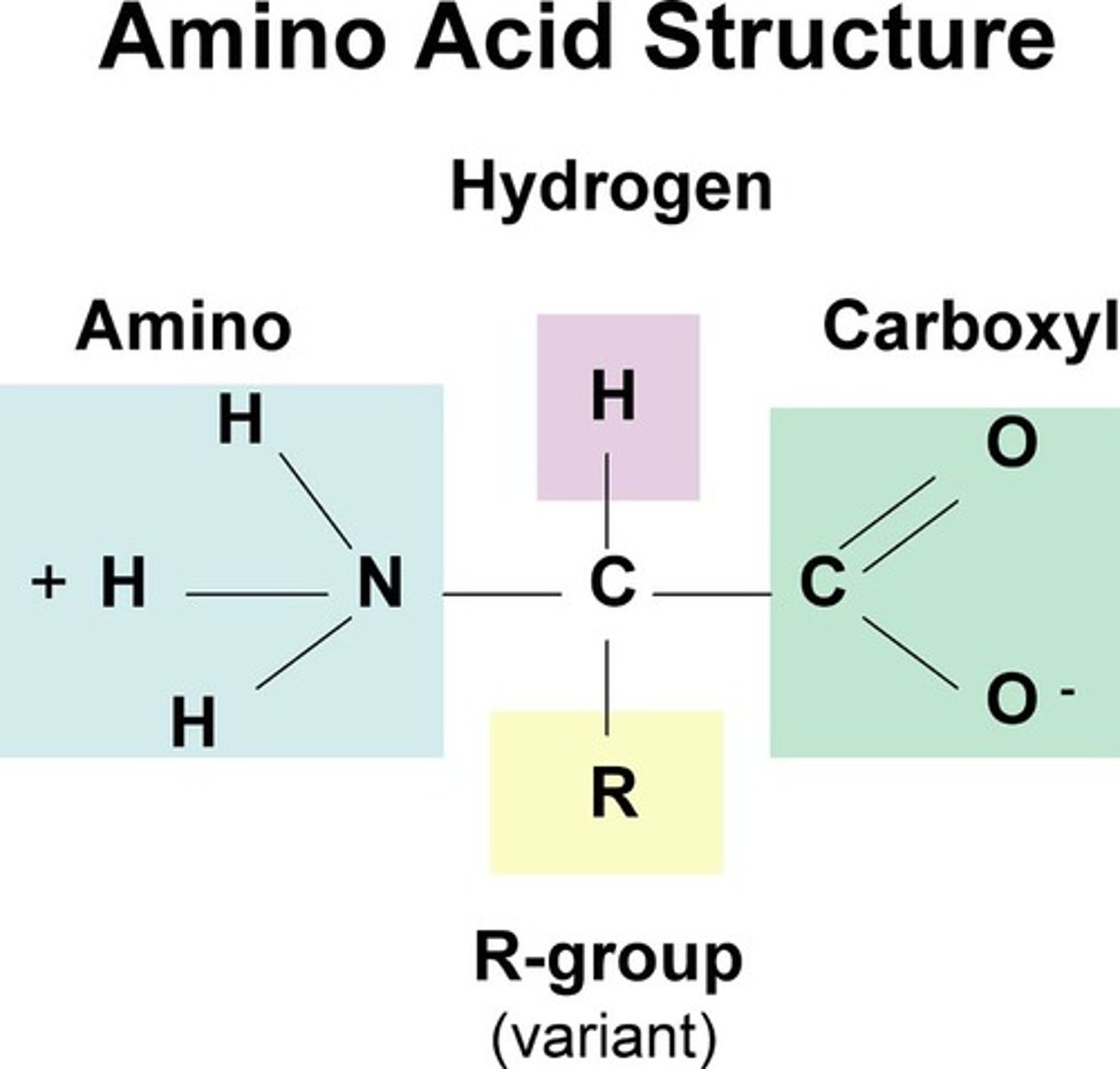
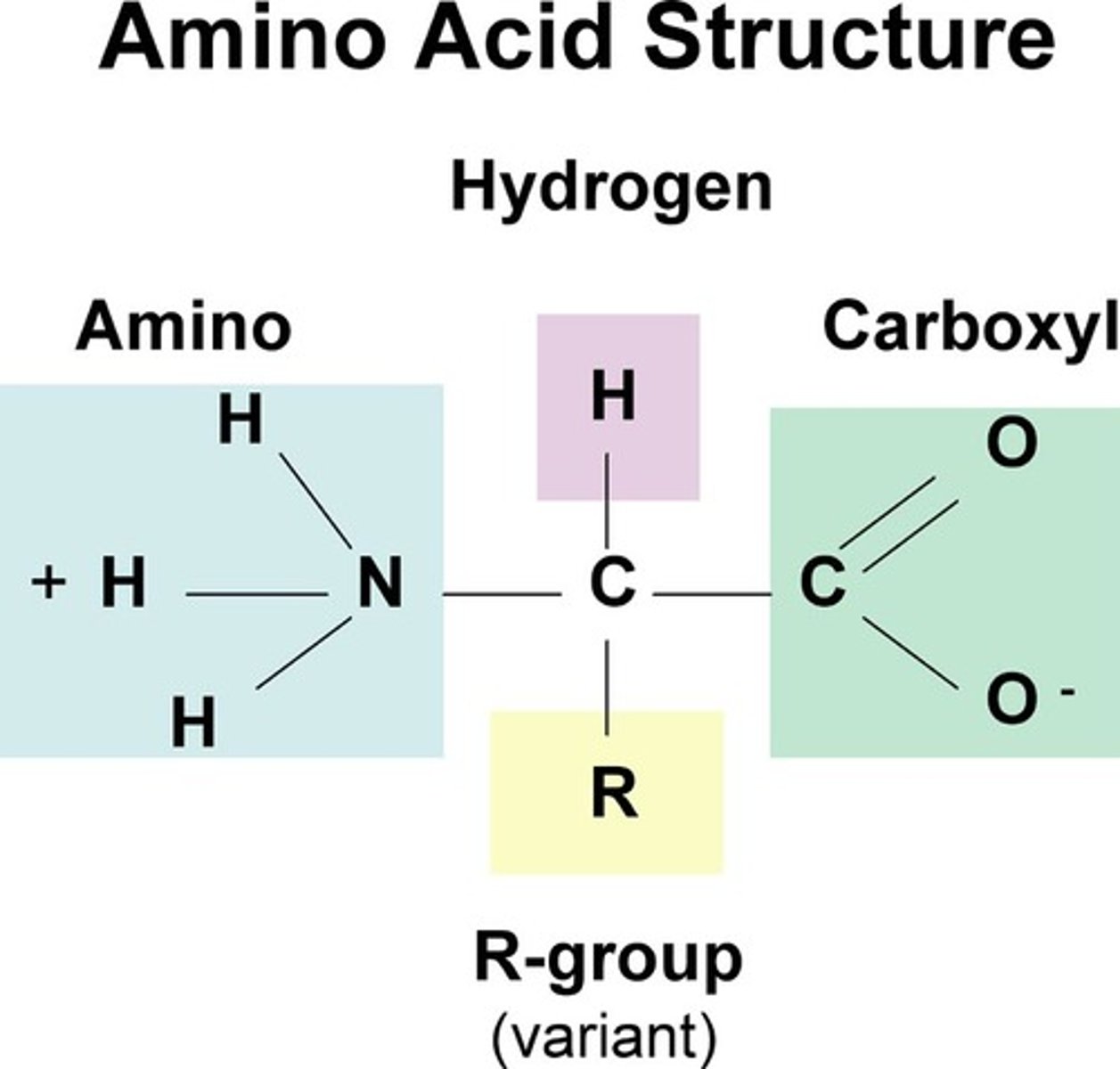
Structure of an amino acid
Amine group, Carboxylic acid group, R group
How are amino acids formed?
Joined by peptide bonds between amine group and carboxyl group in condensation reactions
How can dipeptides/polypeptides be broken down?
Breaking the peptide bonds in hydrolysis
Levels of protein structure
- Primary
- Secondary
- Tertiary
- Quaternary
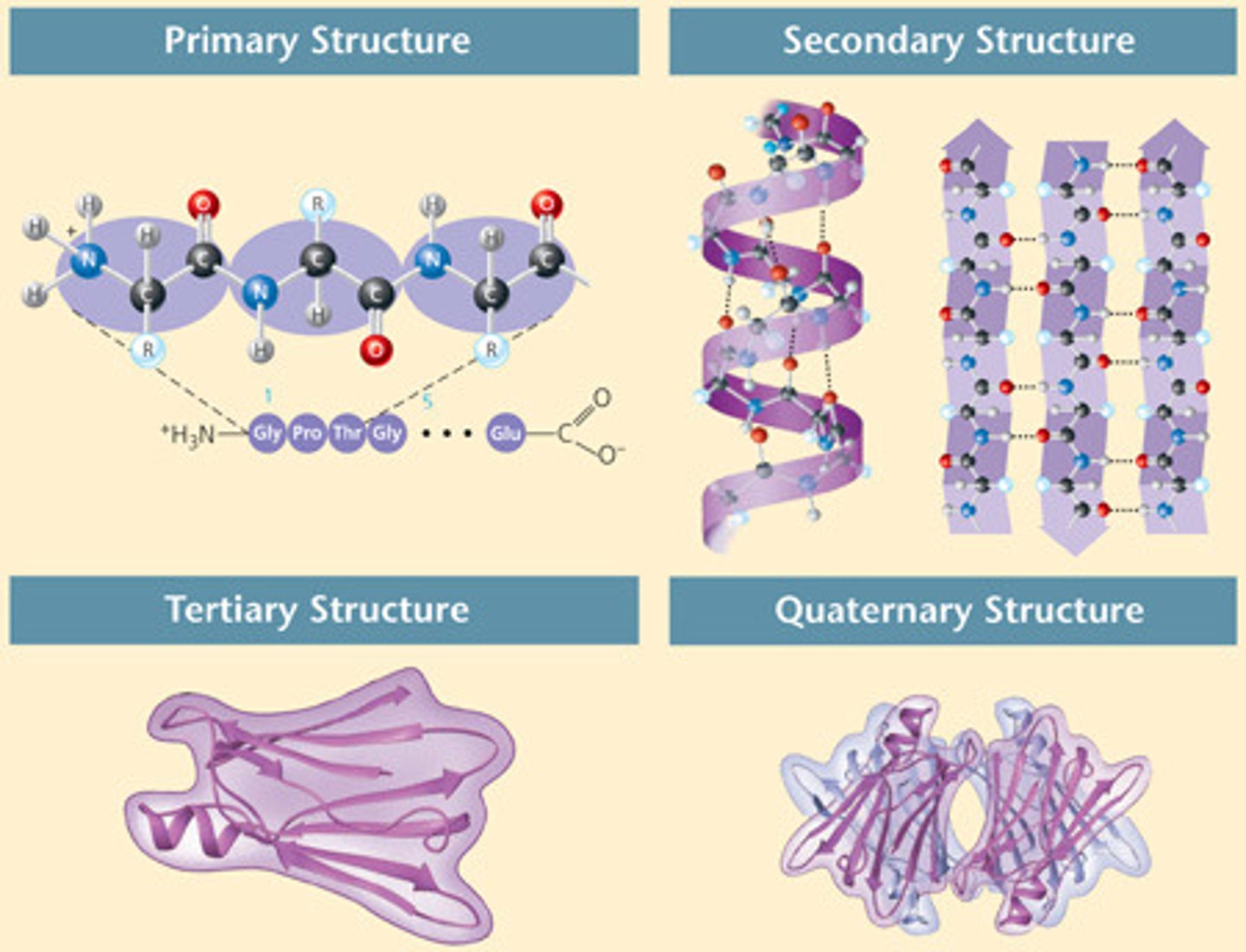
Primary protein structure
Sequence of amino acids in a polypeptide chain
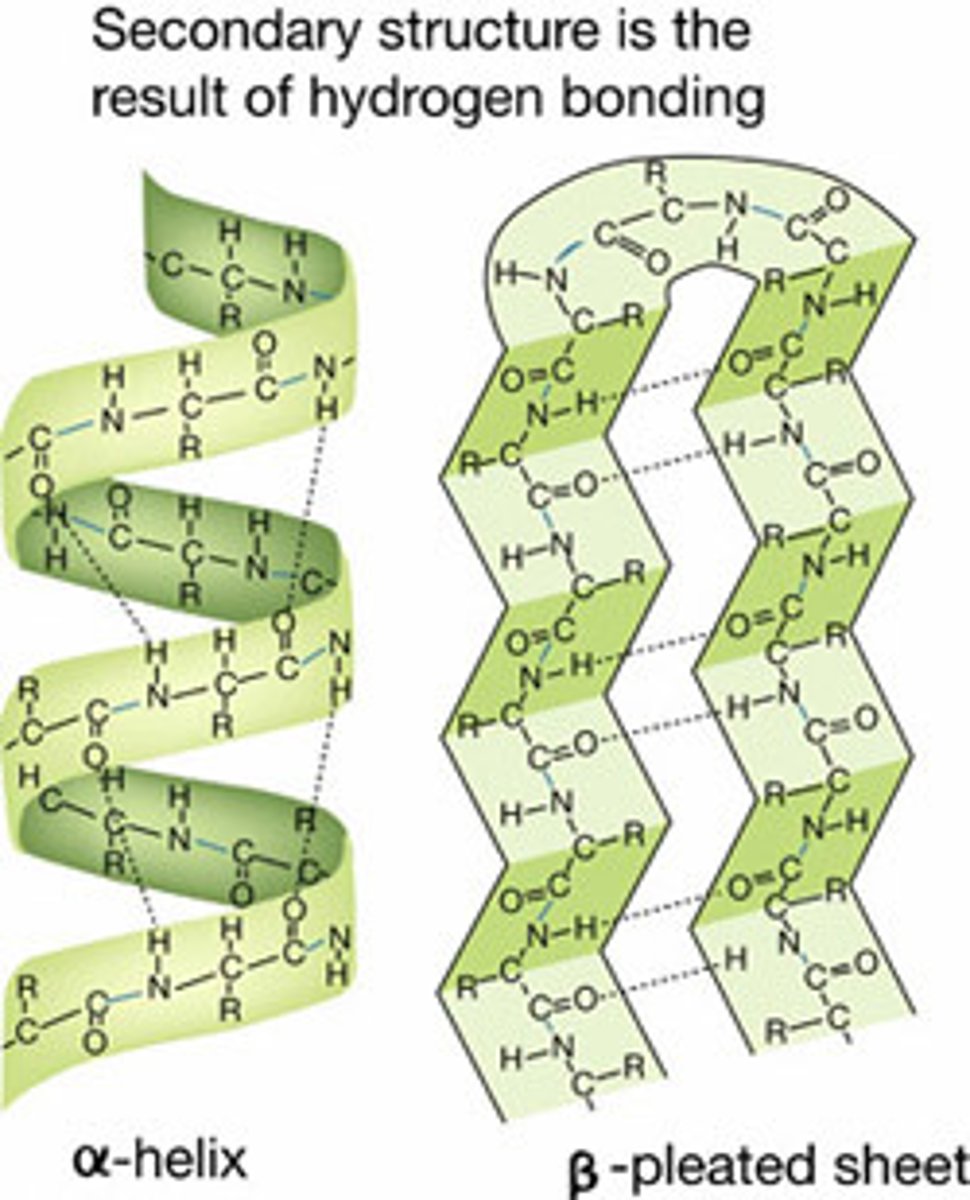
Secondary protein structure
- Alpha helix or Beta pleated sheets
- Hydrogen bonding between amide and carboxyl group
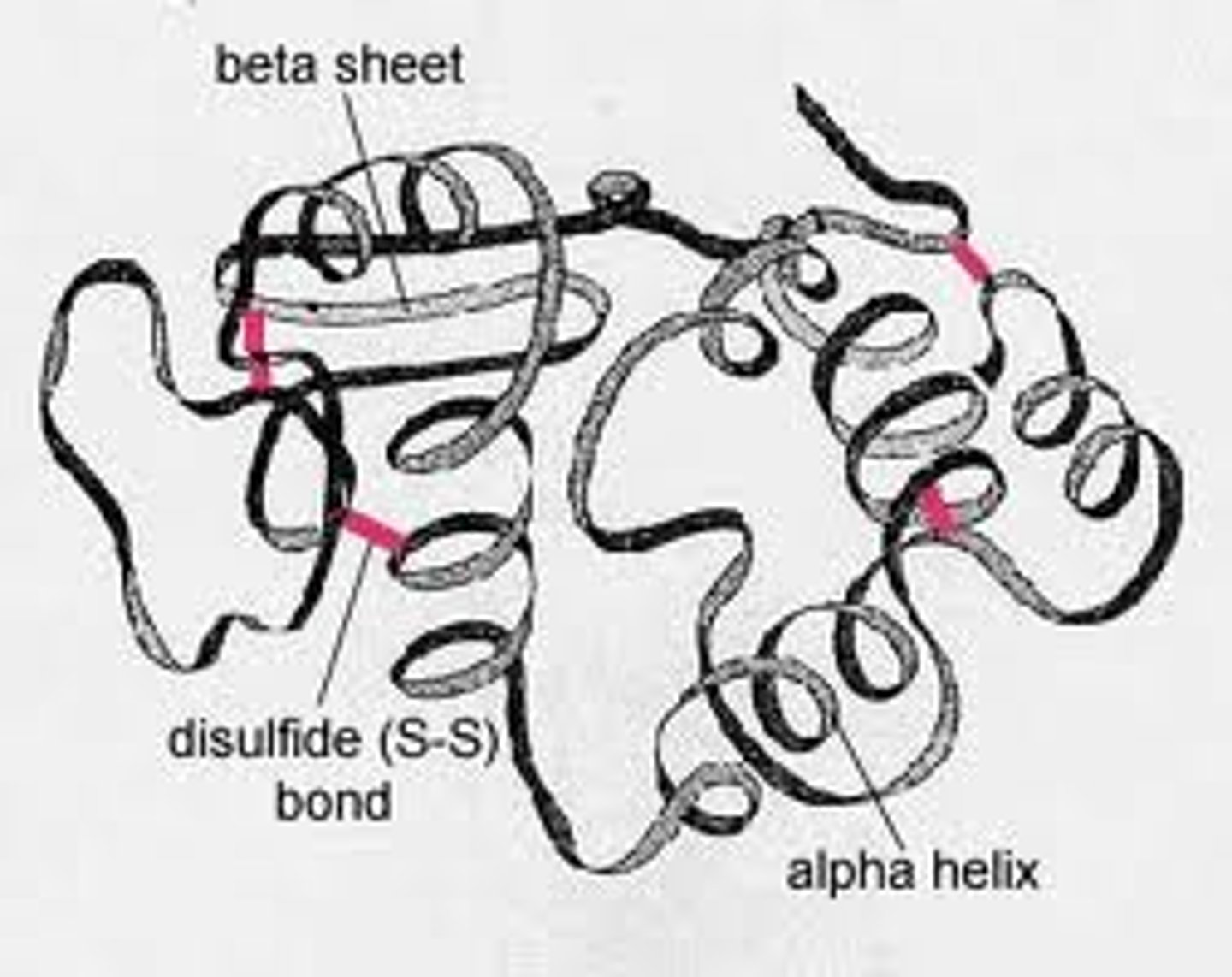
Tertiary protein structure
- 3D shape of protein due to R group interactions
- Hydrophilic/phobic interactions (polar and non polar R groups), hydrogen bonds (weak), disulfide bonds (cysteine) and ionic bonds (between oppositely charged R groups) hold the polypeptides together
Quaternary protein structure
- Protein made of 2+ polypeptide chains
- Each polypeptide is a subunit
Structure of globular proteins
- Compact
- Water soluble (hydrophilic R groups on outside of molecule)
- Spherical
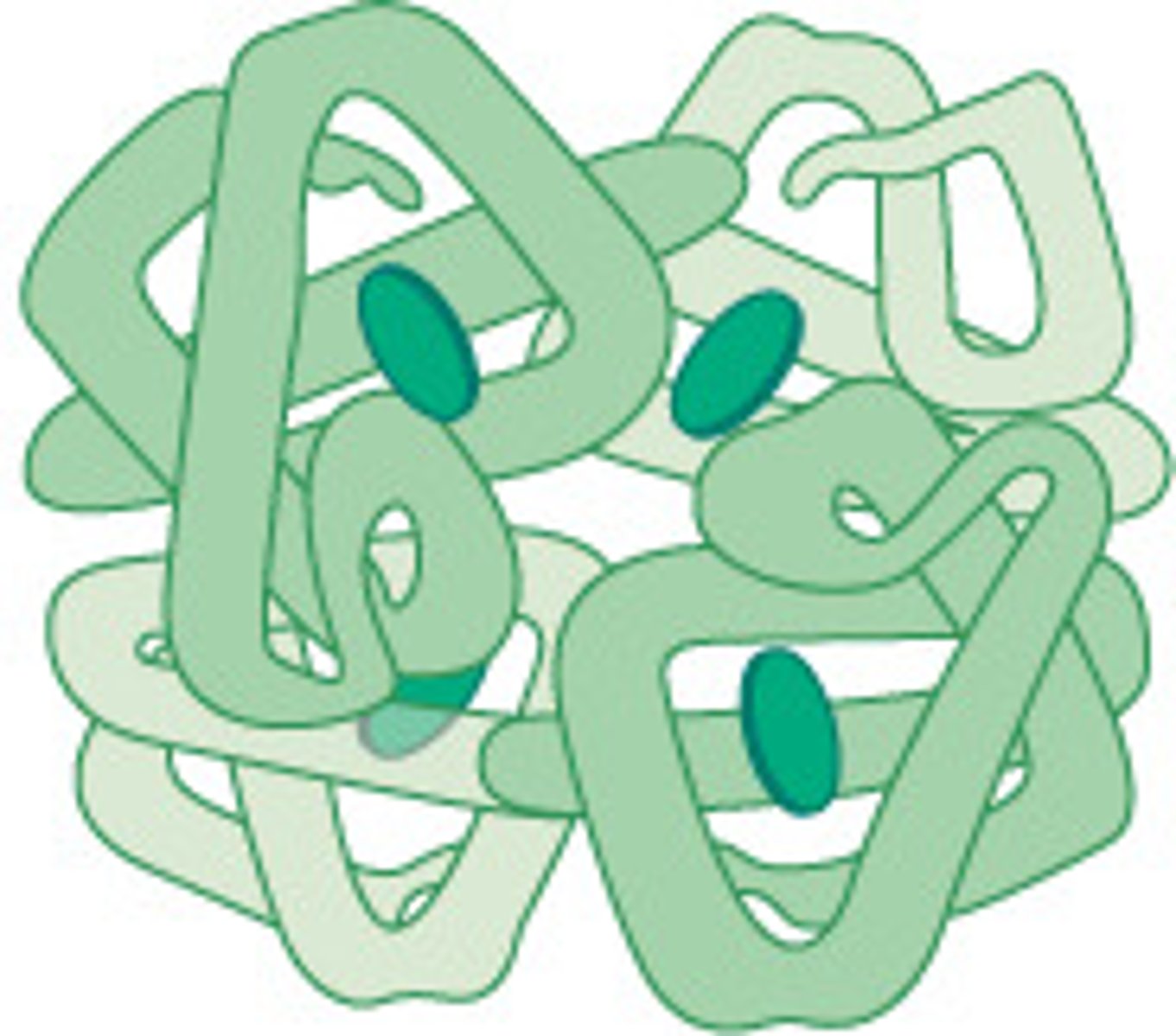
Insulin: Function and Protein type
- Globular protein
- Hormone that regulates blood glucose levels (soluble so transported in blood)
Conjugated protein
Globular proteins with a prosthetic group (non-protein component)
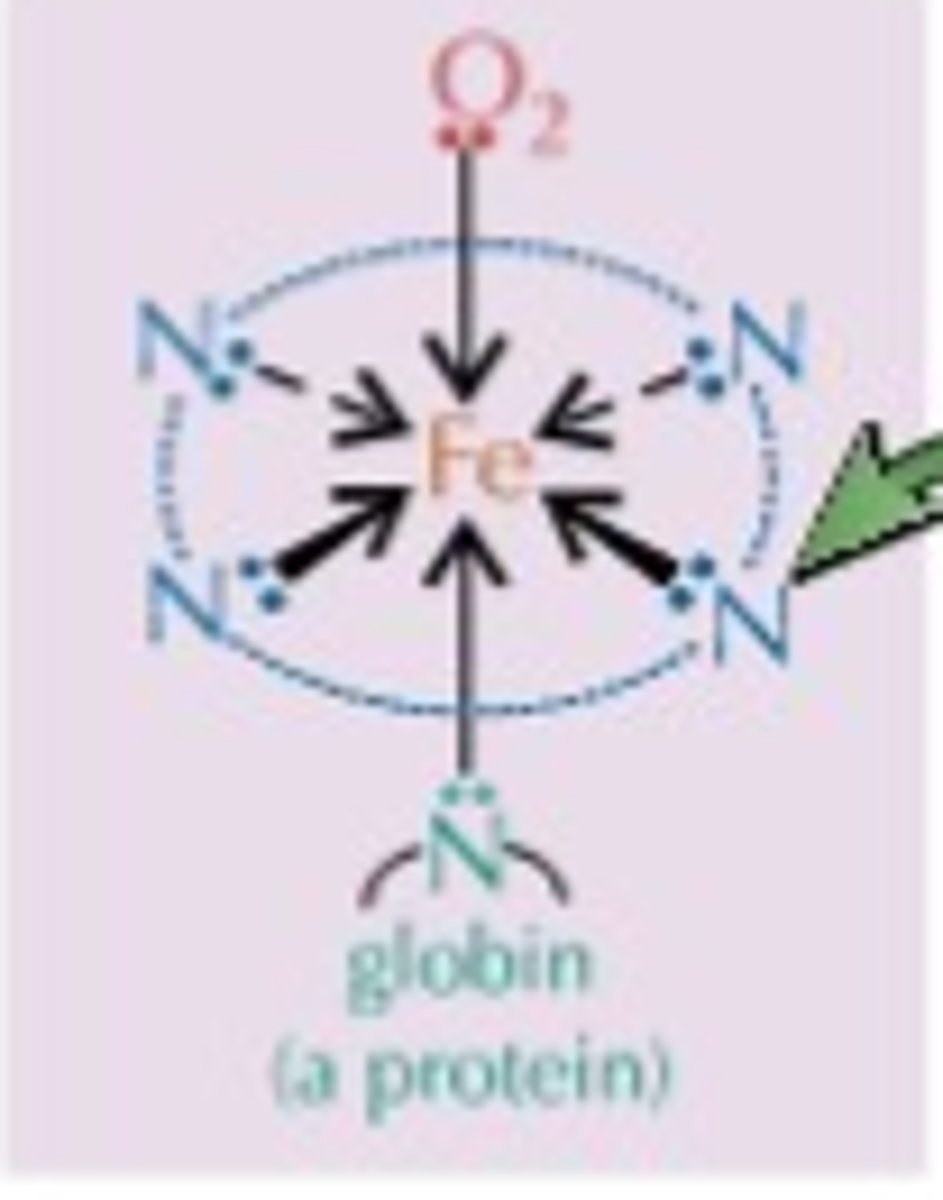
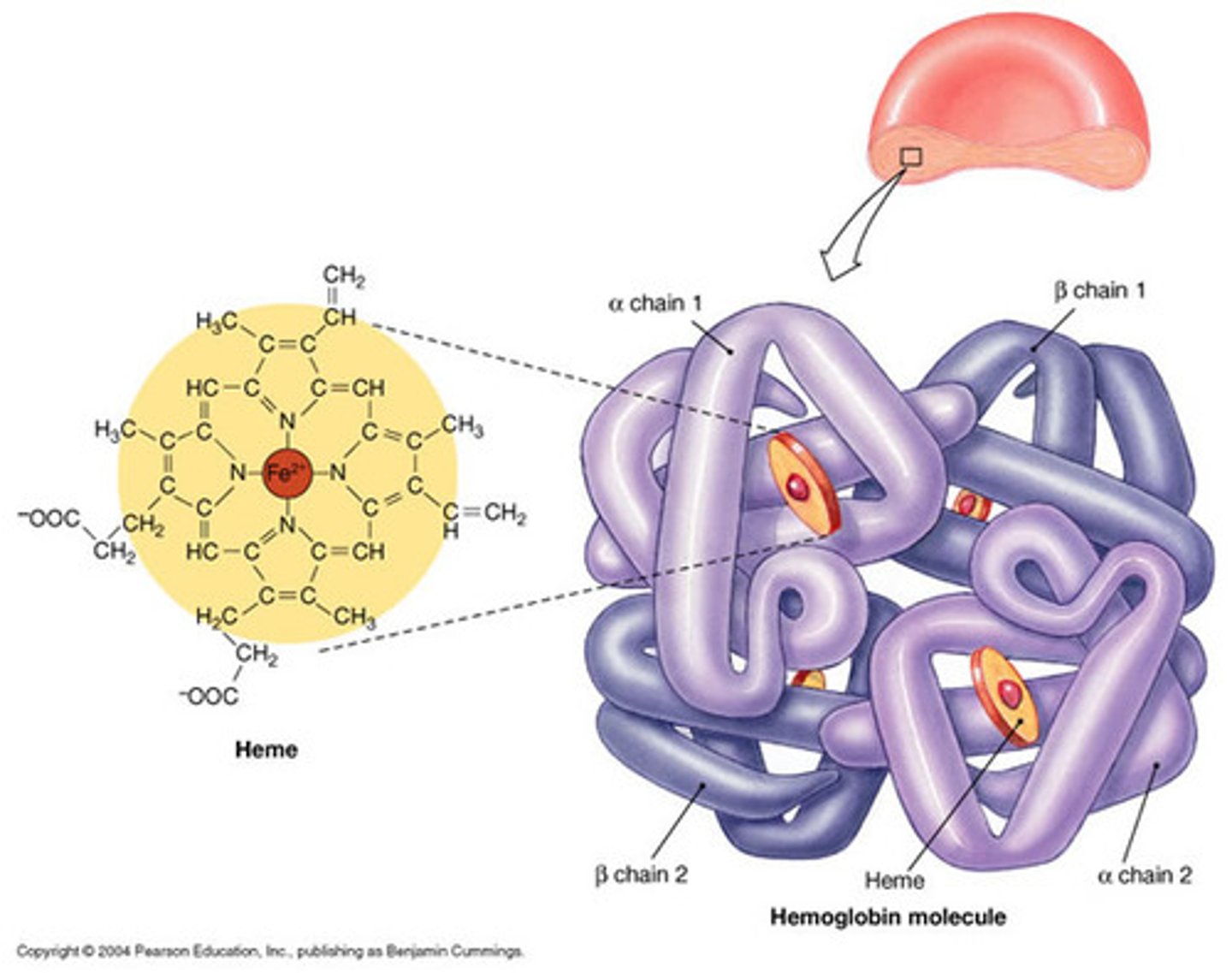
What is a haem group?
- Prosthetic group containing Fe2+ ions
- Catalase and haemoglobin contain haem groups
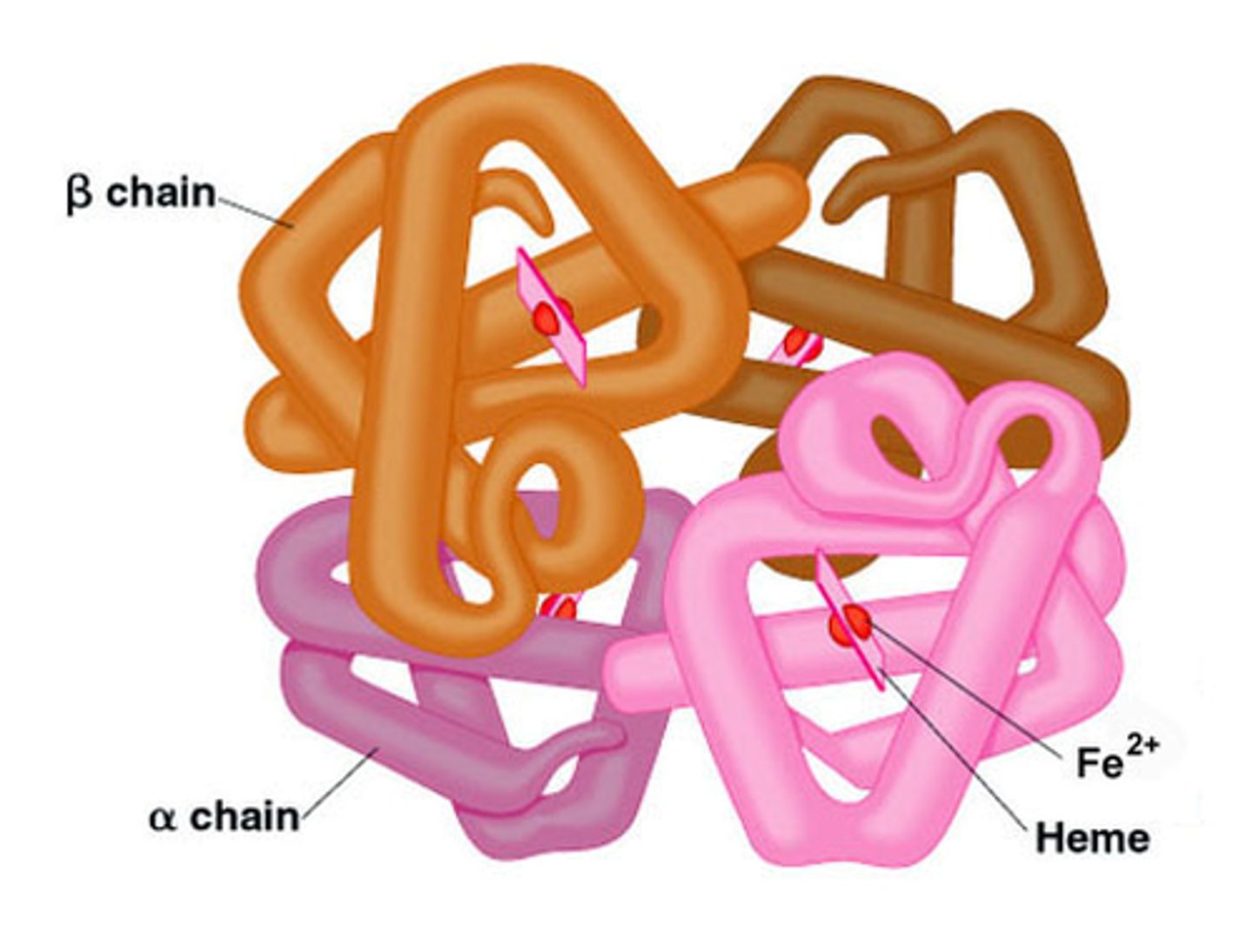
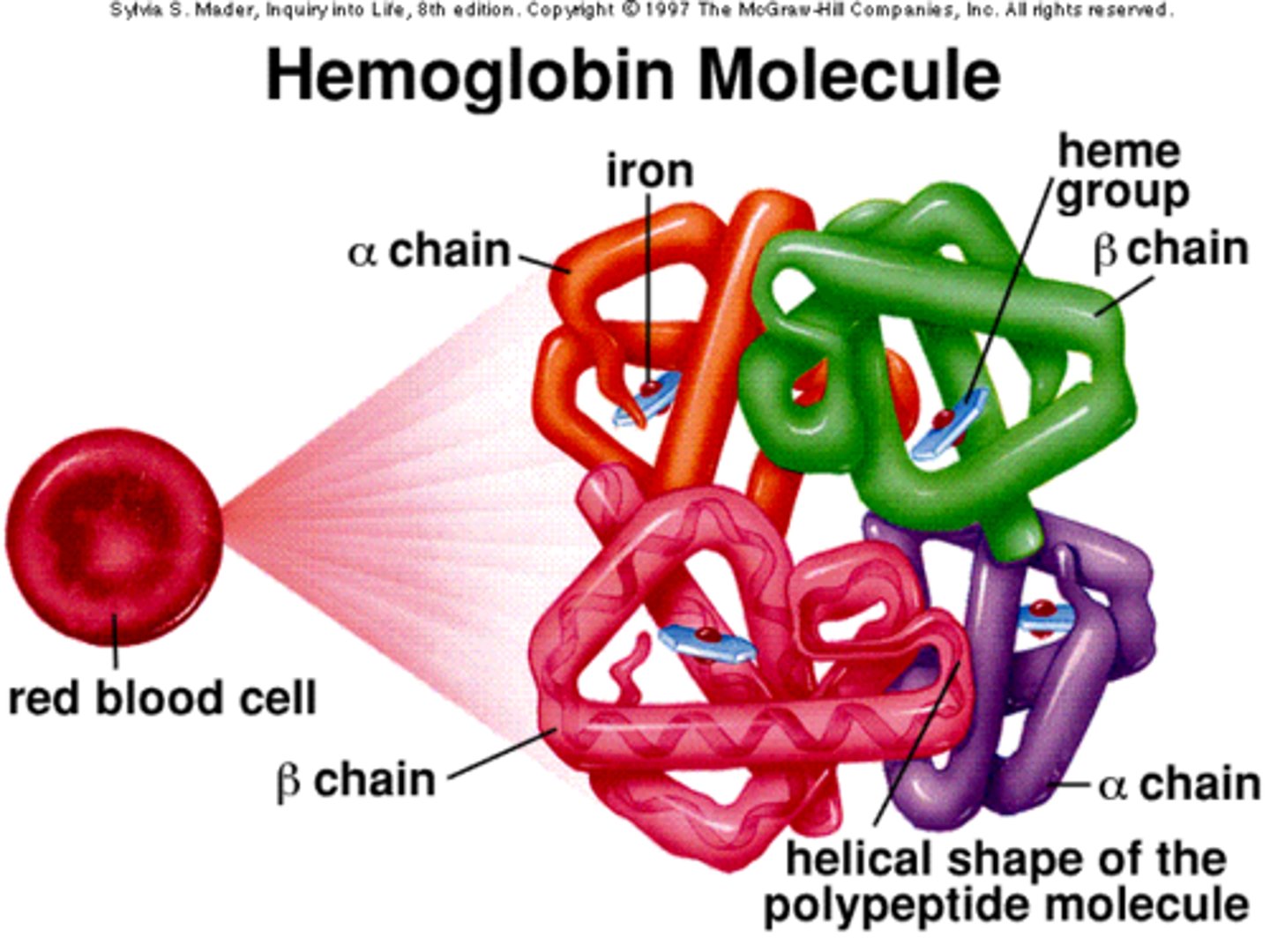
Haemoglobin structure
- Conjugated protein (quaternary)
- Made of 2 alpha and 2 beta subunits (4 polypeptides)
- Has 4 haem groups
- Fe2+ ions combine with oxygen reversibly
- Enables oxygen transport around the body
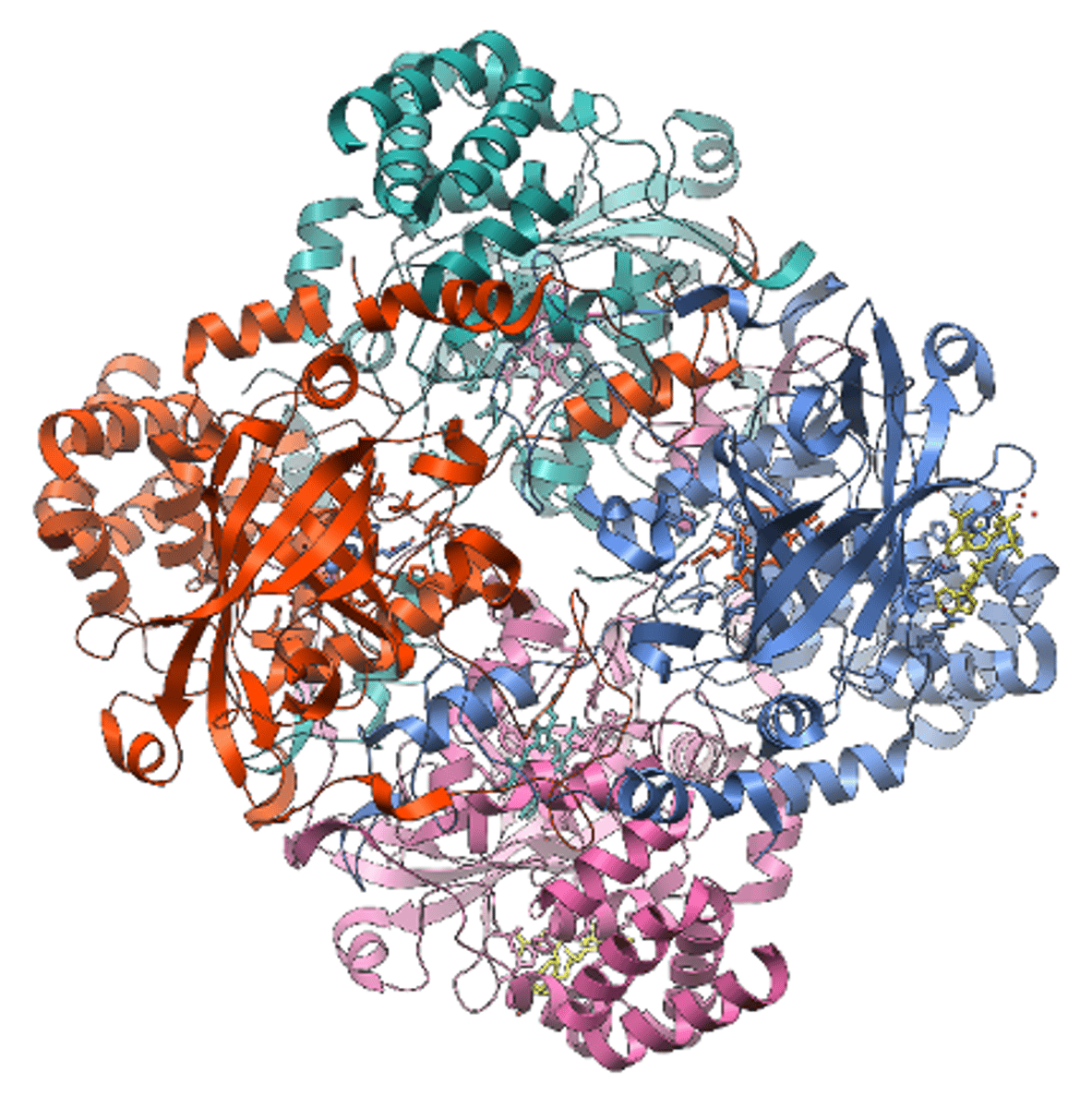
Catalase: Structure and Function
- Conjugated protein (enzyme)
- Has 4 haem groups
- Fe2+ ion allows it to breakdown hydrogen peroxide into water and oxygen (common byproduct of metabolism which is damaging to cells)
Properties of Fibrous proteins
- Strong, Long
- Insoluble (many hydrophobic R groups)
- Unreactive
- Organised structure (repetitive amino acid sequence)
Function of Fibrous proteins
Provides structure
Keratin: Structure and Function
- Fibrous protein
- Many cysteine and disulfide bridges
- Found in hair, skin, nails

Elastin: Structure and Function
- Fibrous protein
- Allows tissue to stretch and return to normal size
- Found in elastic fibres (e.g. walls of alveoli)
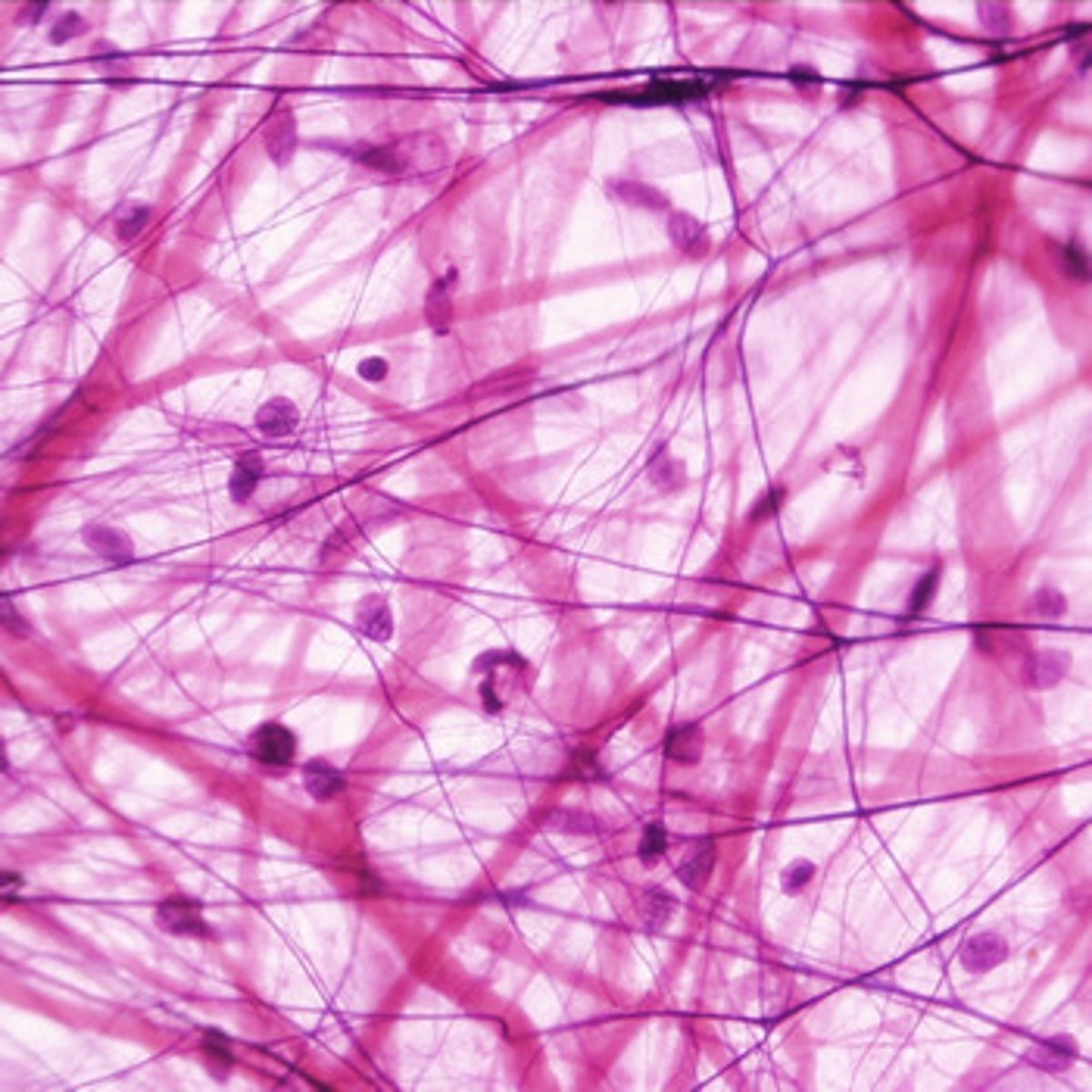
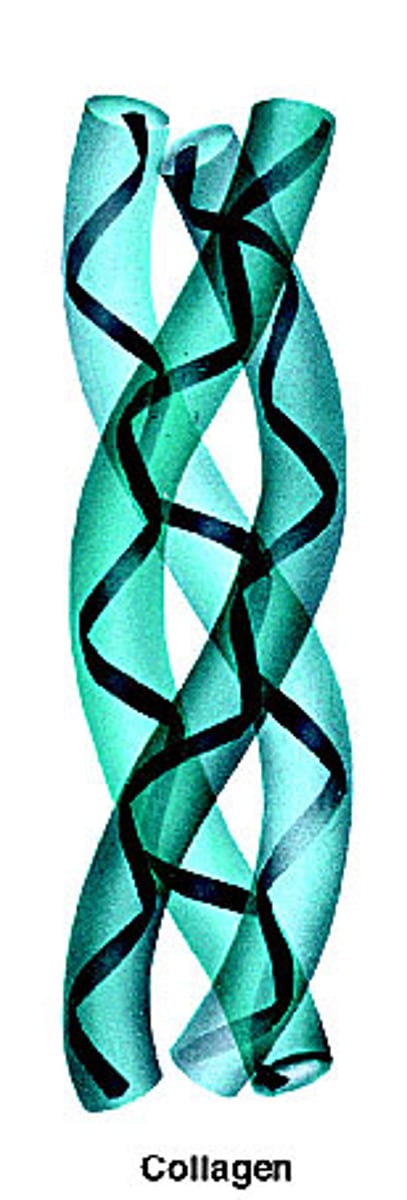
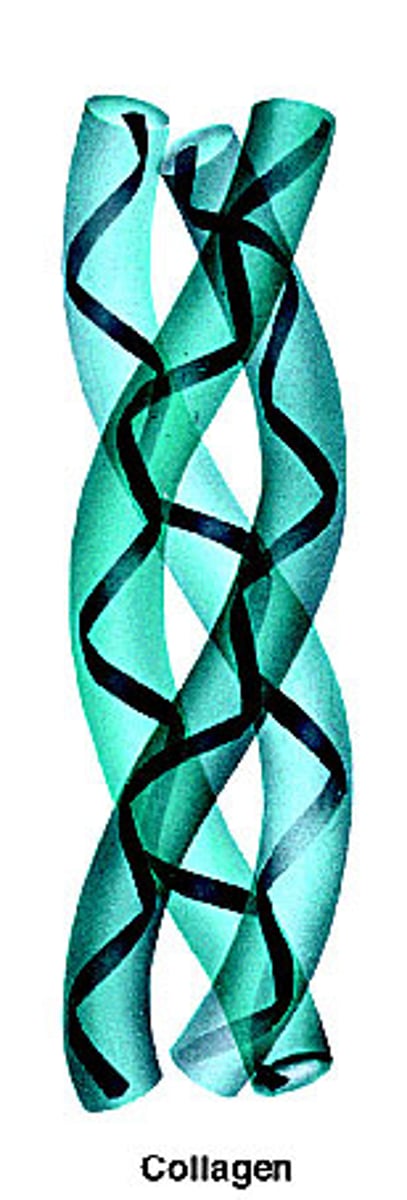
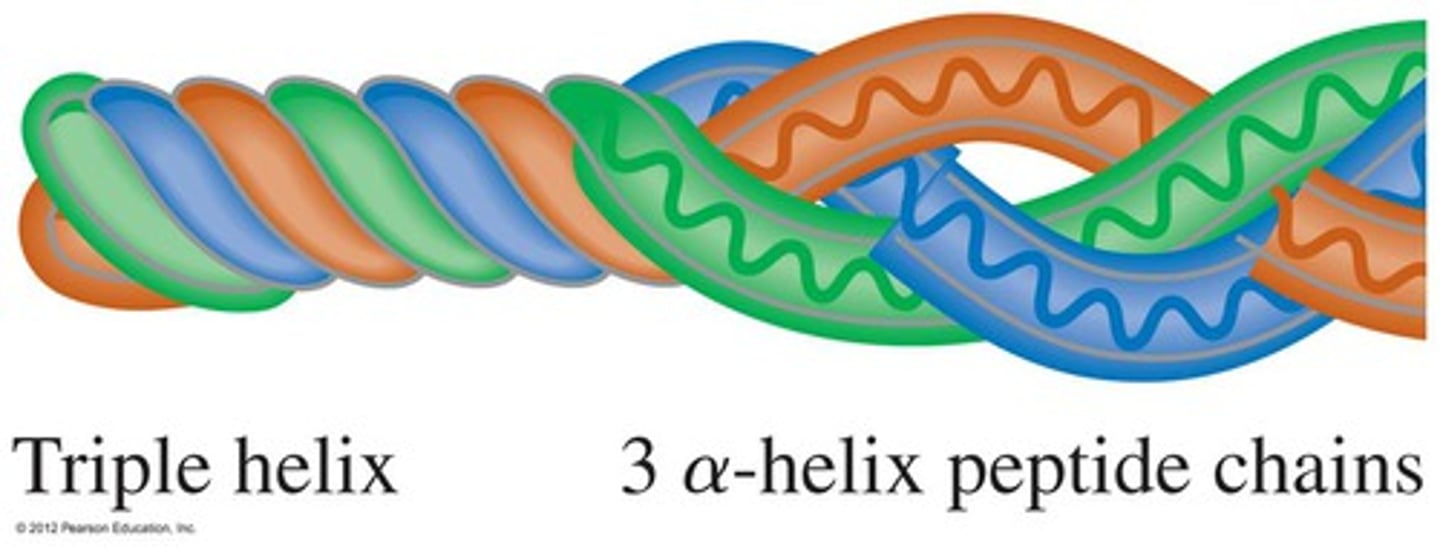
Collagen: Structure and Function
- Fibrous protein
- 3 polypeptides wound together
- Found in skin, tendons, ligaments, nervous system
Calcium ions function (Ca²⁺)
- Nerve impulse transmission
- Muscle contraction
Sodium ions function (Na⁺)
- Nerve impulse transmission
- Regulates cell water potential
- Kidney function
Potassium ions function (K⁺)
- Nerve impulse transmission
- Regulates cell water potential
- Stomatal opening
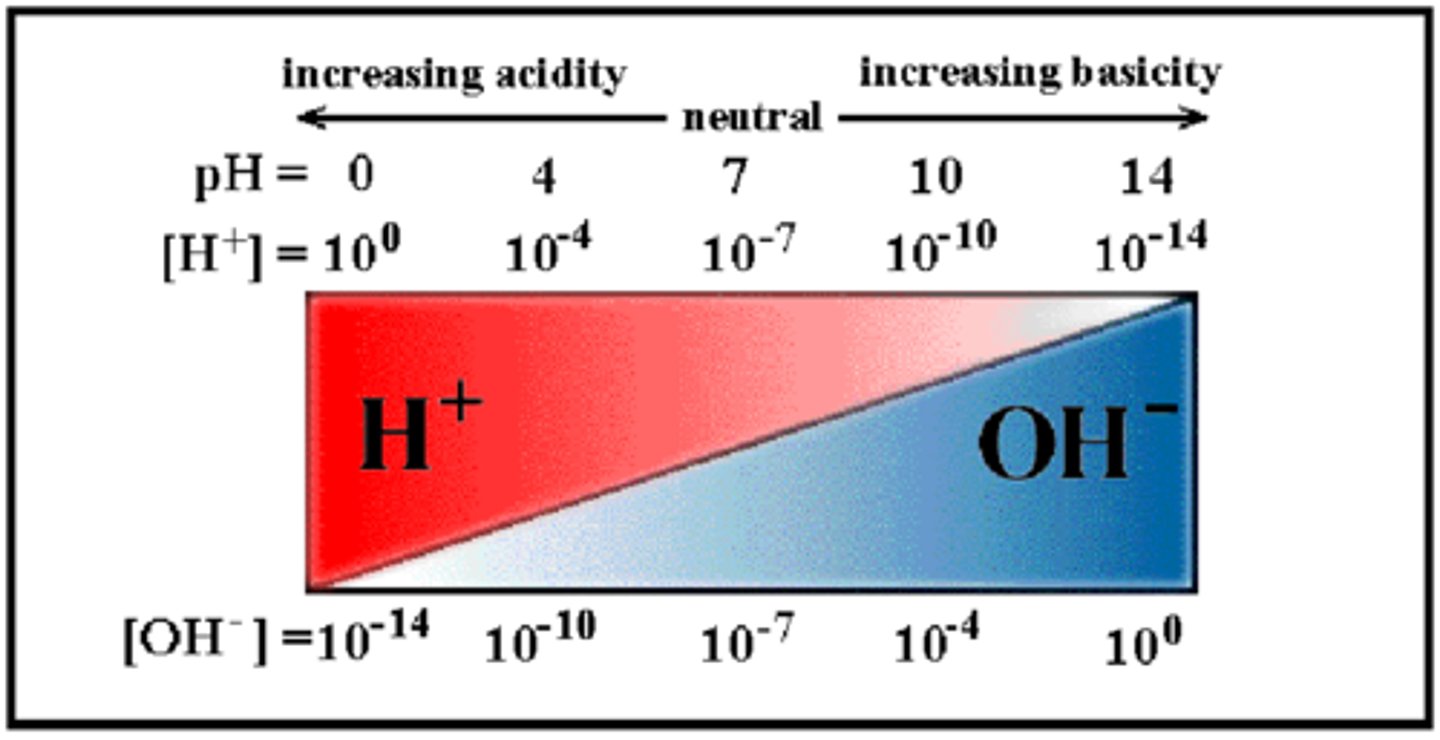
Hydrogen ions function (H⁺)
- Catalyses reactions
- Determines pH
Ammonium ions function (NH₄⁺)
Produces nitrate ions in bacteria
How soluble are Lipids?
- Soluble in organic solvents (eg alcohol)
- Insoluble in water (doesn't affect water potential)

Function of Lipids
- Energy storage (releases water too)
- Insulation
- Protection (saturated/hard)

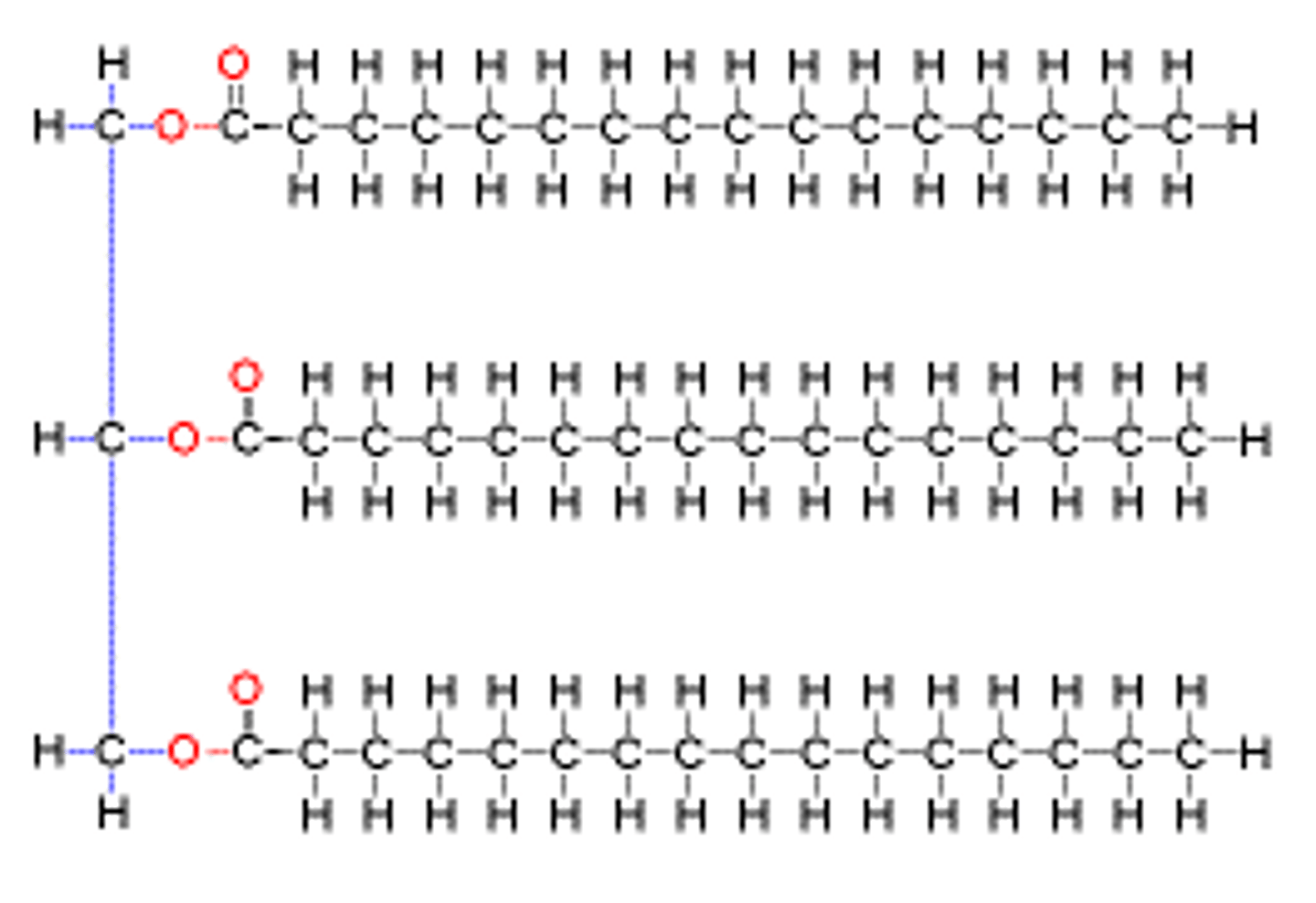
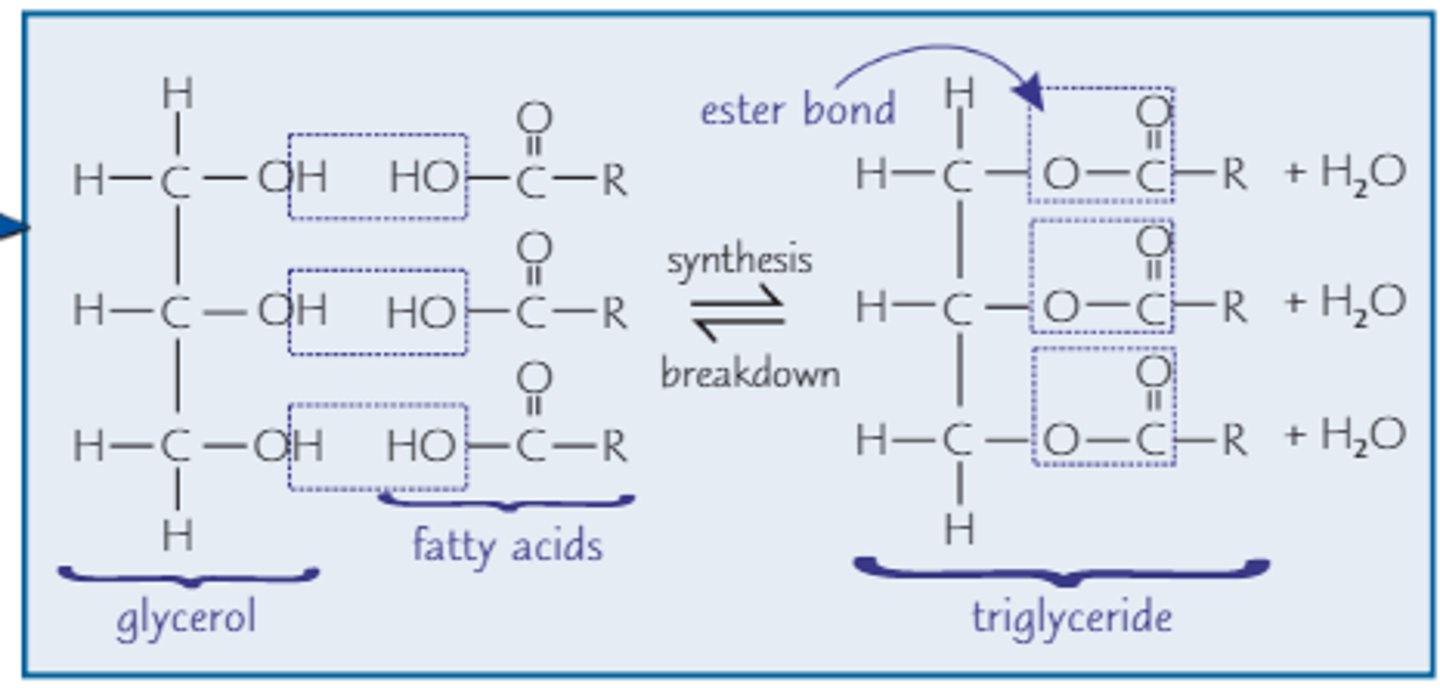
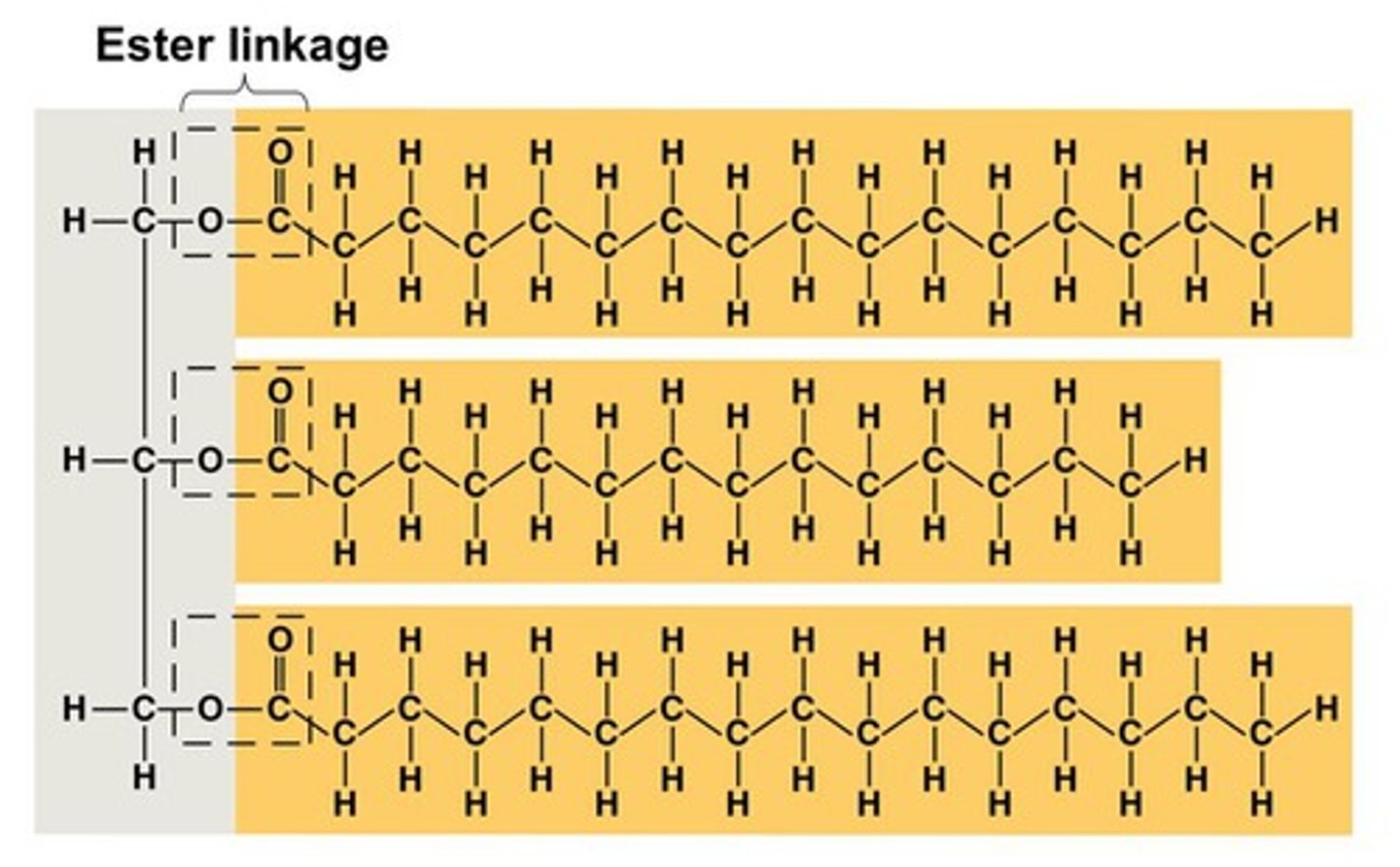
Trigylceride structure
1 glycerol covalently bonded with 3 fatty acids by ester linkage
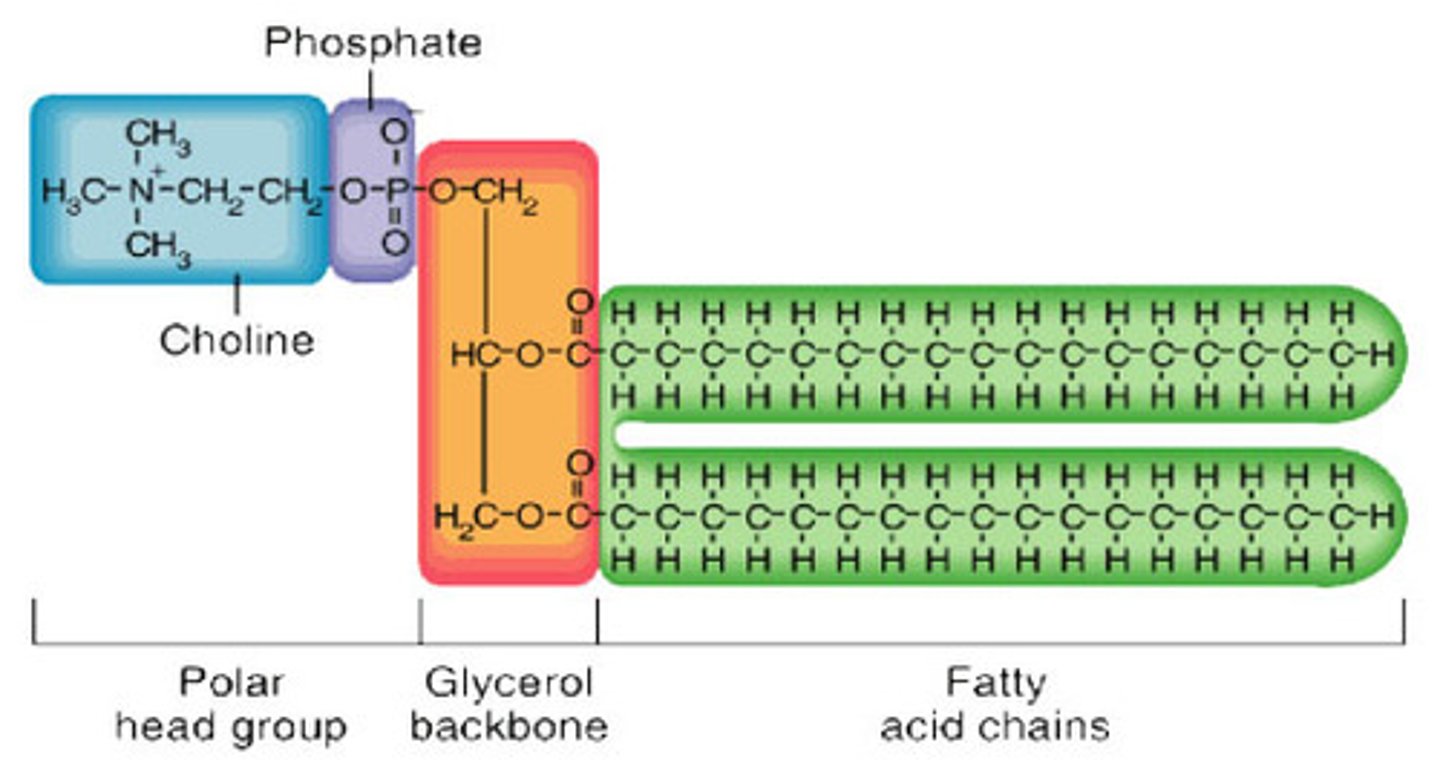
Structure of Phospholipids
- 1 phosphate head (hydrophilic)
- 2 fatty acids (hydrophobic tails)
- 1 glycerol
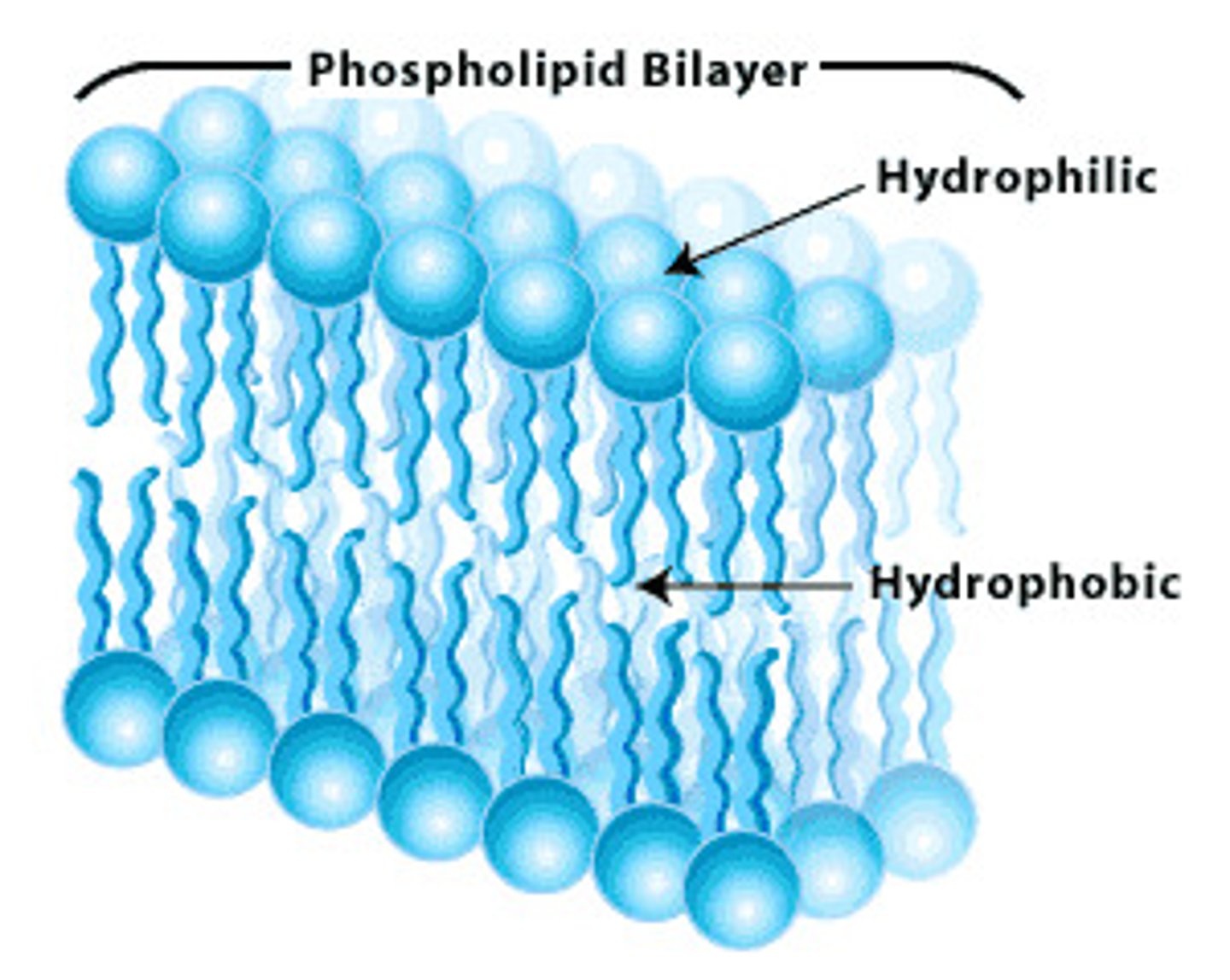
Function of Phospholipids
- Forms the phospholipid bilayer in water
- Which makes up cell membranes
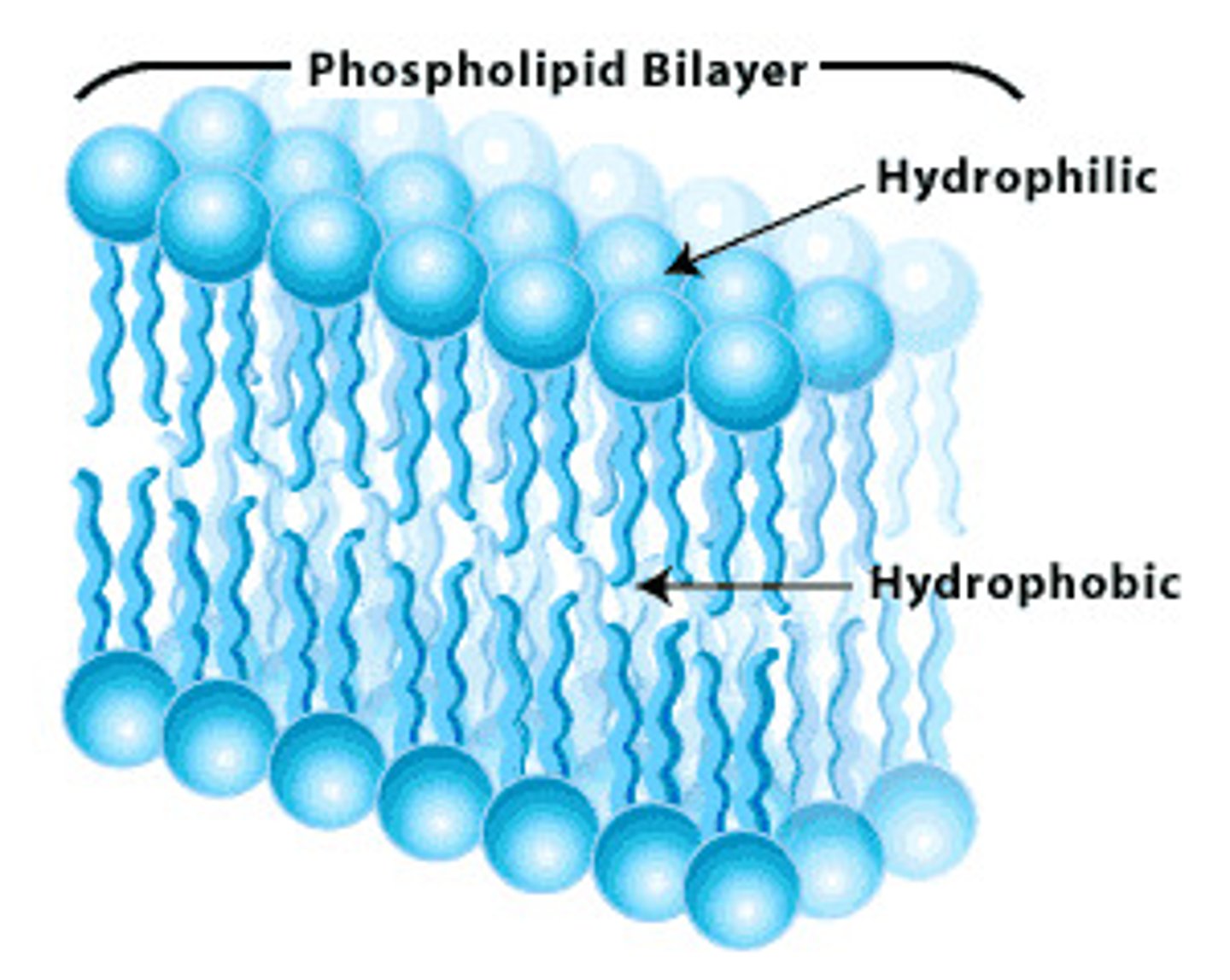
How do phospholipids form the bilayer of plasma membranes?
- Phosphate head is hydrophilic and fatty acid tails are hydrophobic
- Hydrophobic tails are repelled by water outside plasma membrane
- Hydrophilic head forms H bonds with water
- Tails face inwards towards each other because they're hydrophobic
What is a Macromolecule?
Large molecule formed by smaller organic molecules (eg trigylcerides)
Difference between the structure of saturated fats and unsaturated fats
Saturated fats - single carbon bonds
Unsaturated fats - double carbon bonds
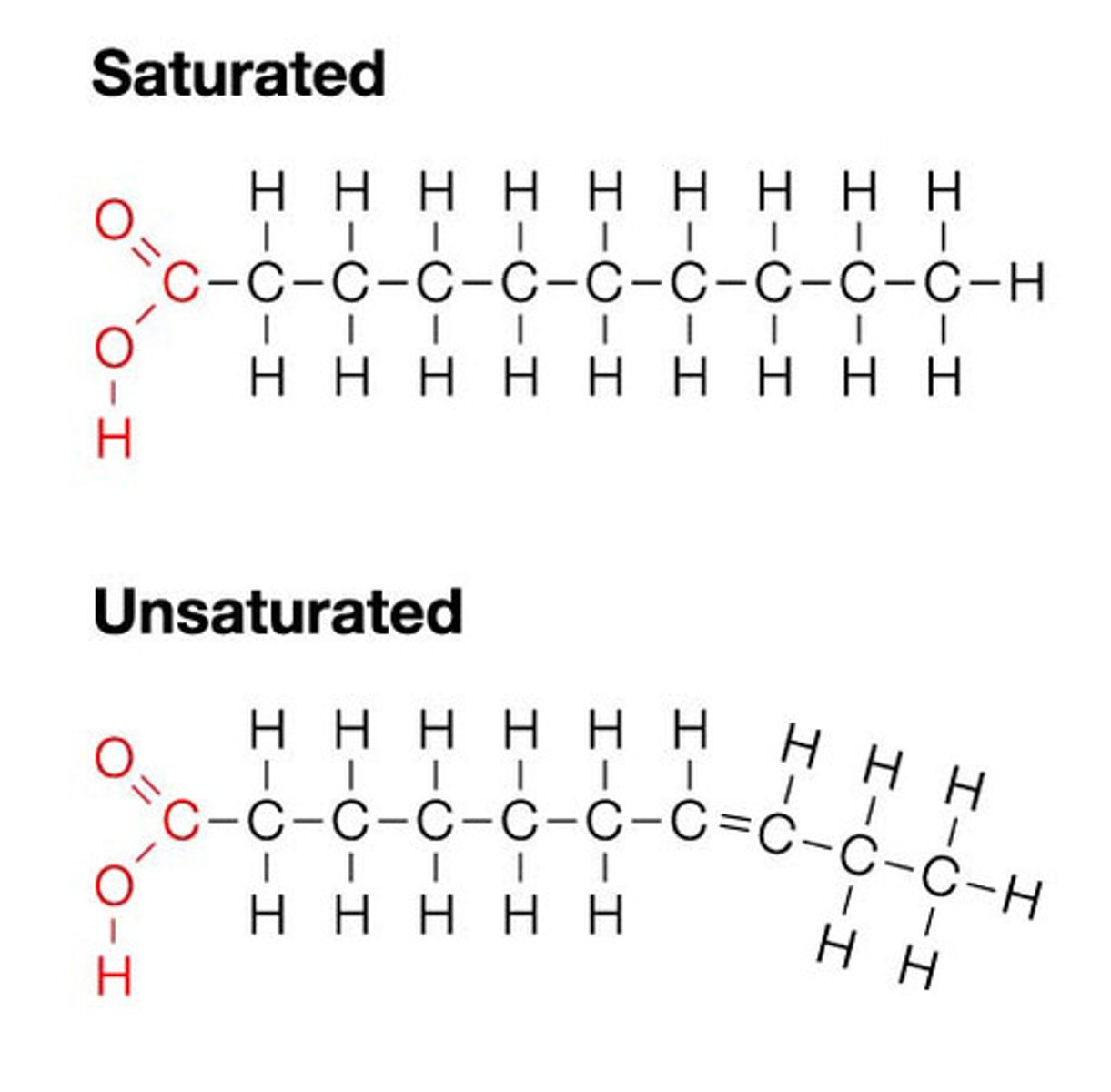
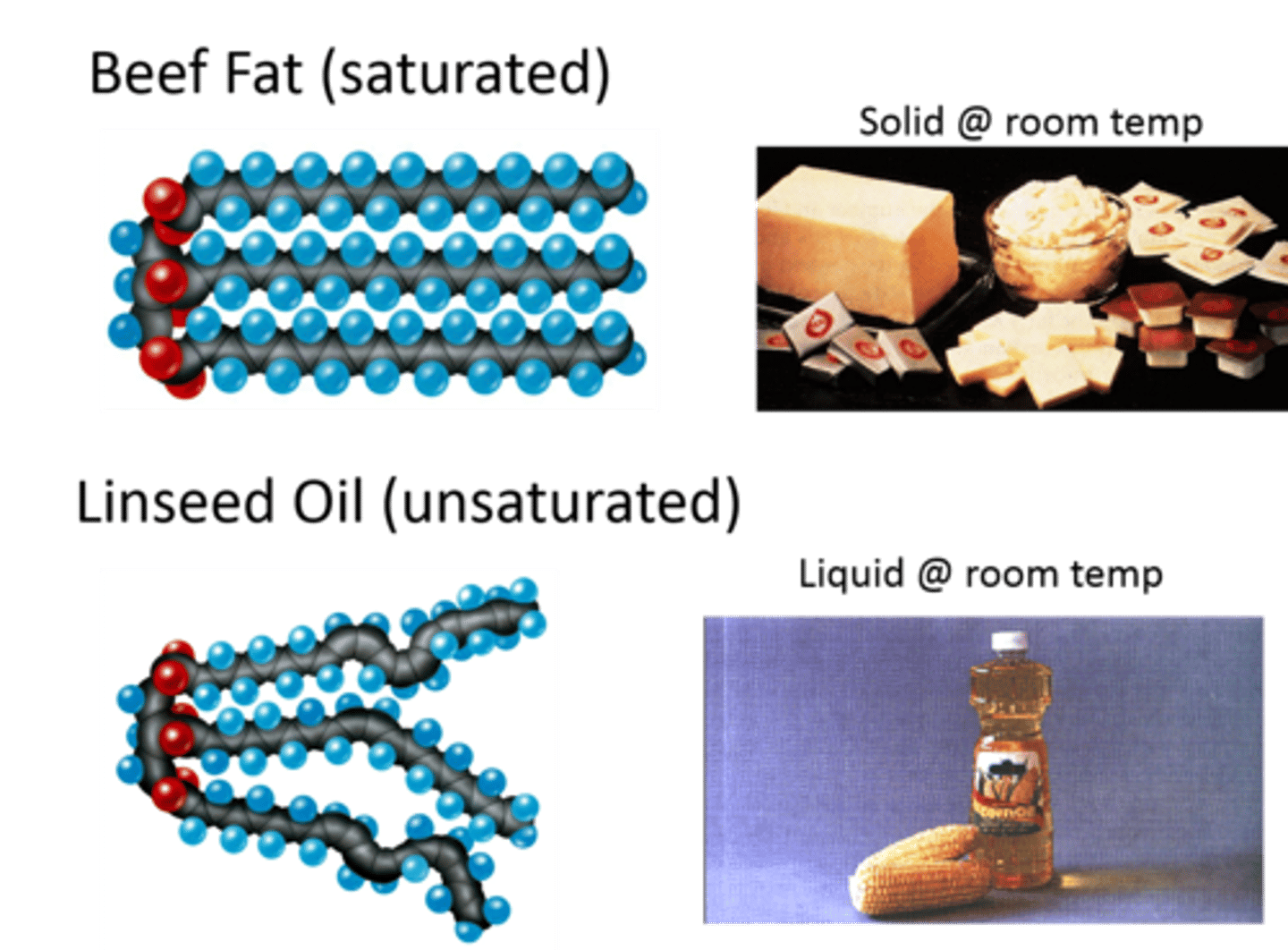
Why are unsaturated fats liquid at room temperature?
- More unsaturated (double) bonds which bend
- Can't pack closely together
- So weaker intermolecular bonds
- So lower melting point
- So liquid at RT
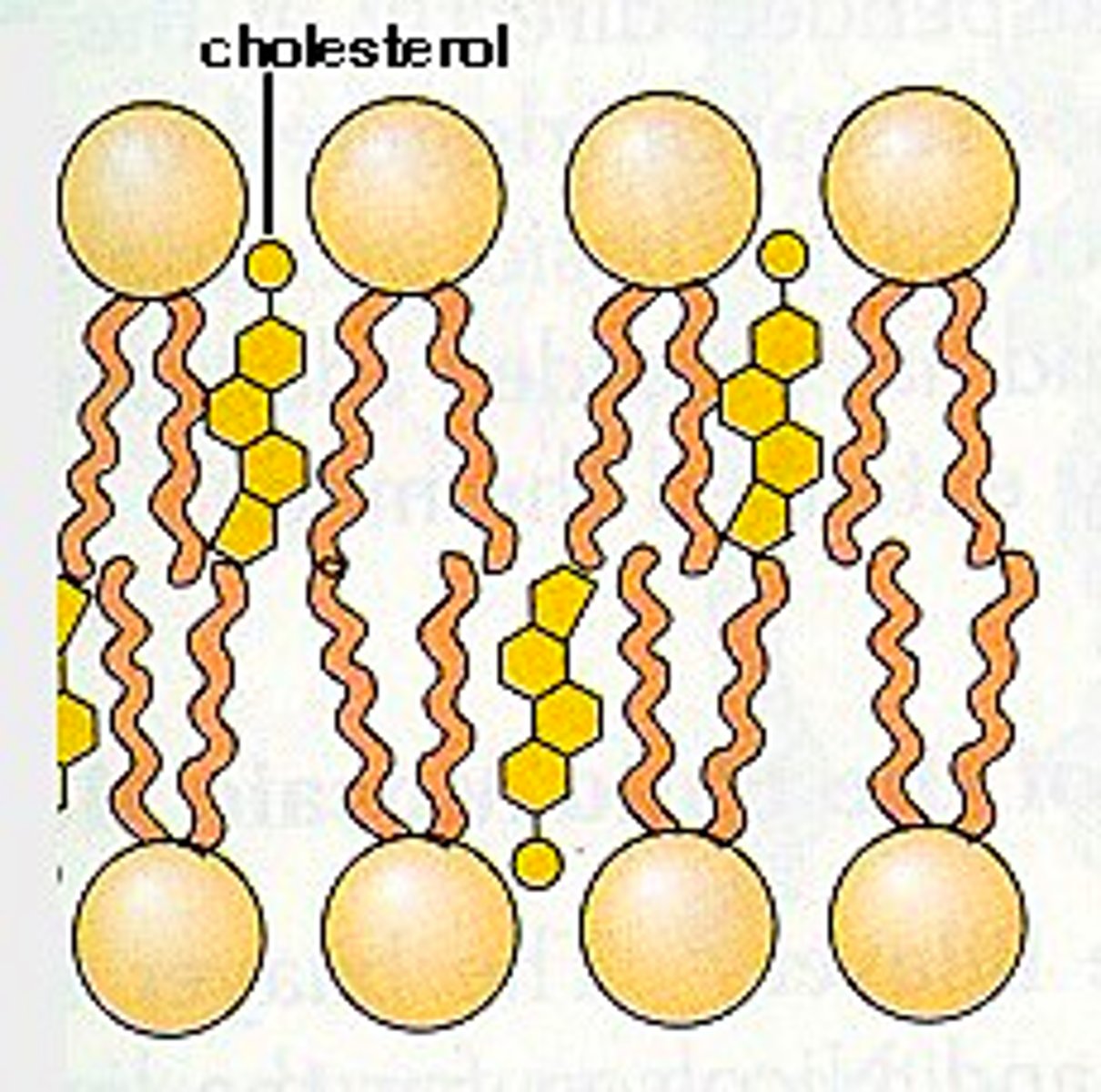
Cholesterol: Structure and Function
- Steroid of 4 carbon rings
- Hydrophobic and hydrophiliic regions
- Adds stability to phospholipid bilayer
- Affects membrane fluidity
How are triglycerides formed?
Condensation reaction forming ester bonds
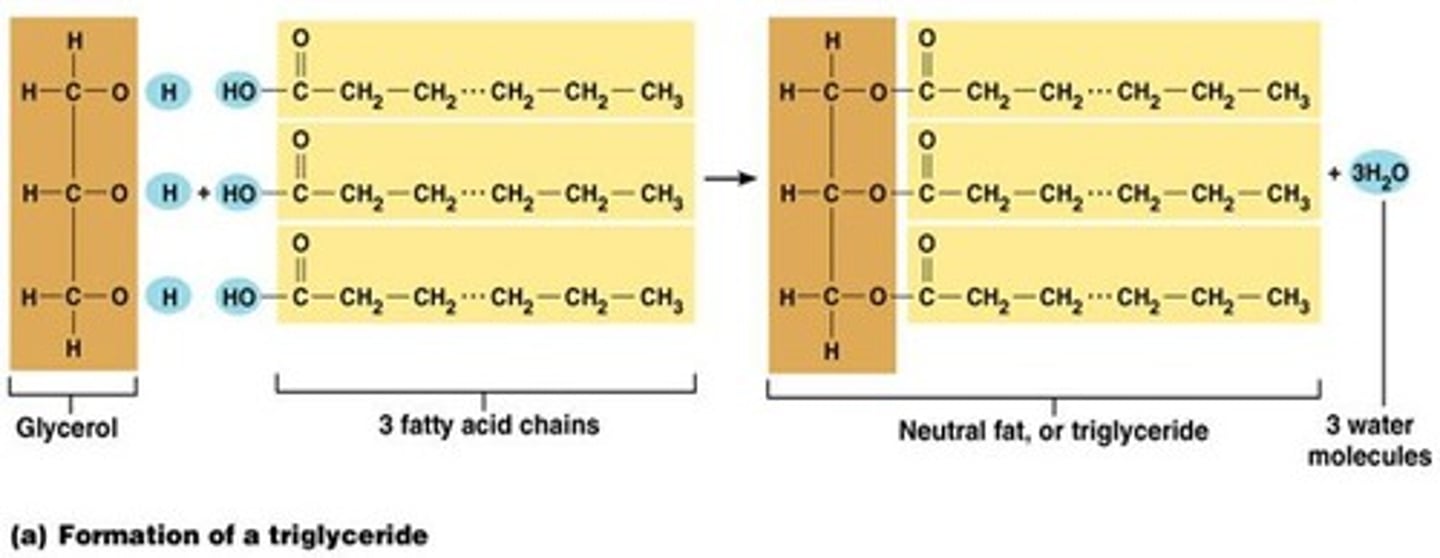
How are triglycerides broken down?
Hydrolysis reaction breaking ester bonds
Why are triglycerides used as energy reserves?
They store more energy than carbohydrates due to their hydrocarbon chains
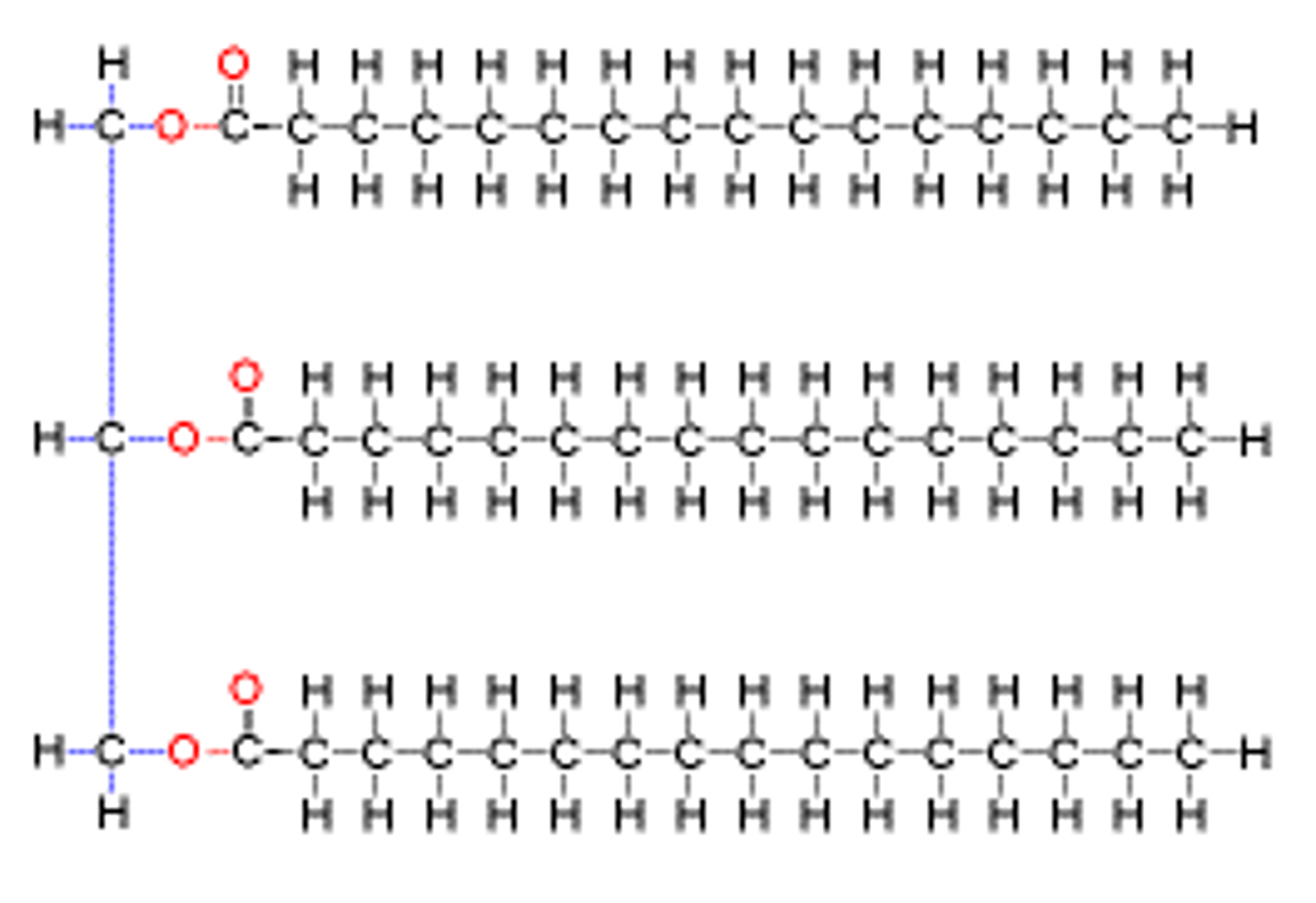
What is an ester bond?
- COO
How are solid samples prepared for chemical tests?
Crush sample and dissolve in distilled water
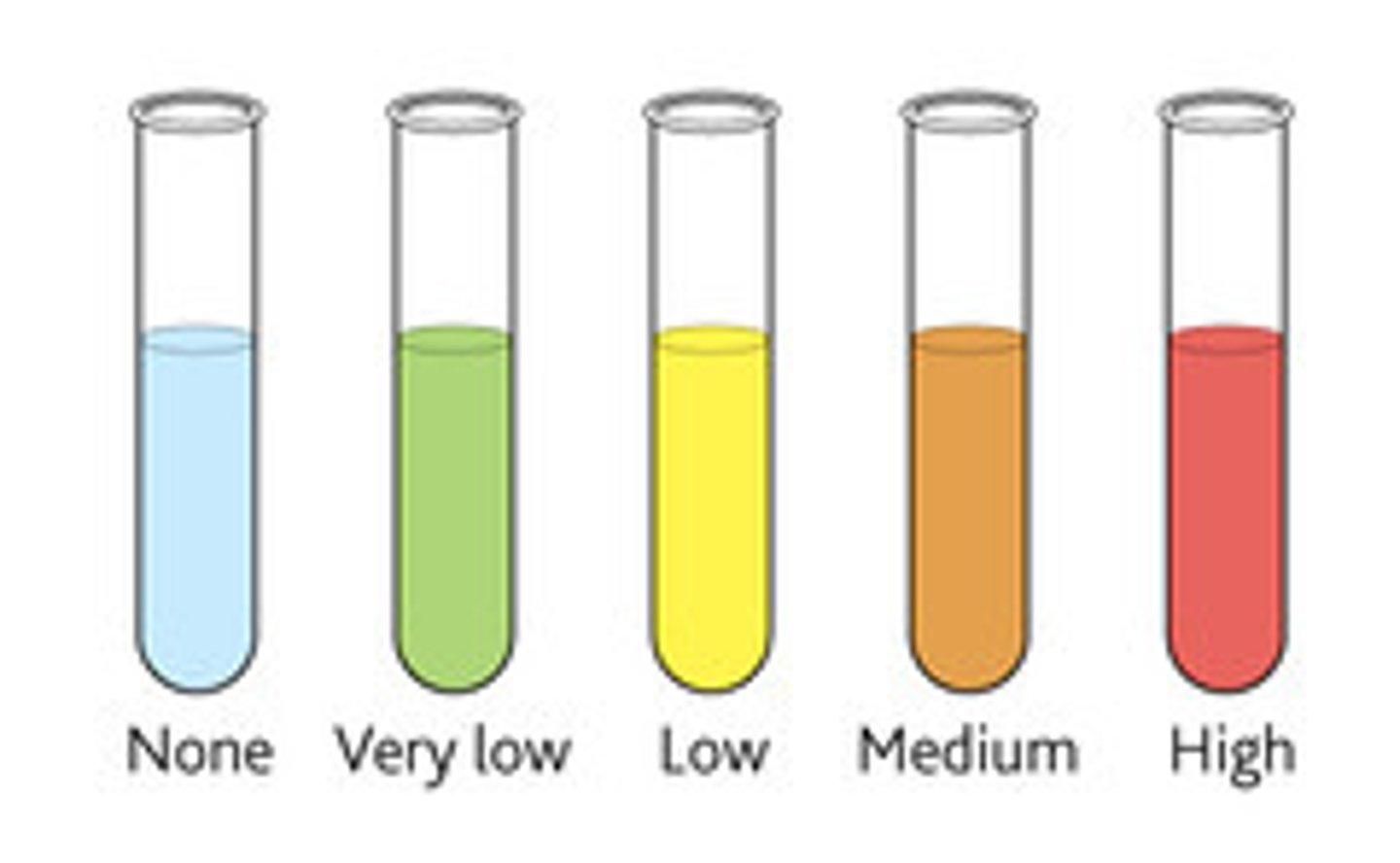
Reducing sugars test
- Benedict's solution
- Heat for 5 mins at 80°C
- Blue -> Brick-red precipitate
Non-reducing sugars test
Following a negative Benedicts test (stays blue):
- Add HCl acid and boil
- Neutralise by adding NaOH
- Add Benedict's solution and heat for 5 mins at 80°C
- Blue -> Brick-red precipitate
Proteins test
- Biuret's solution
- Blue -> Purple

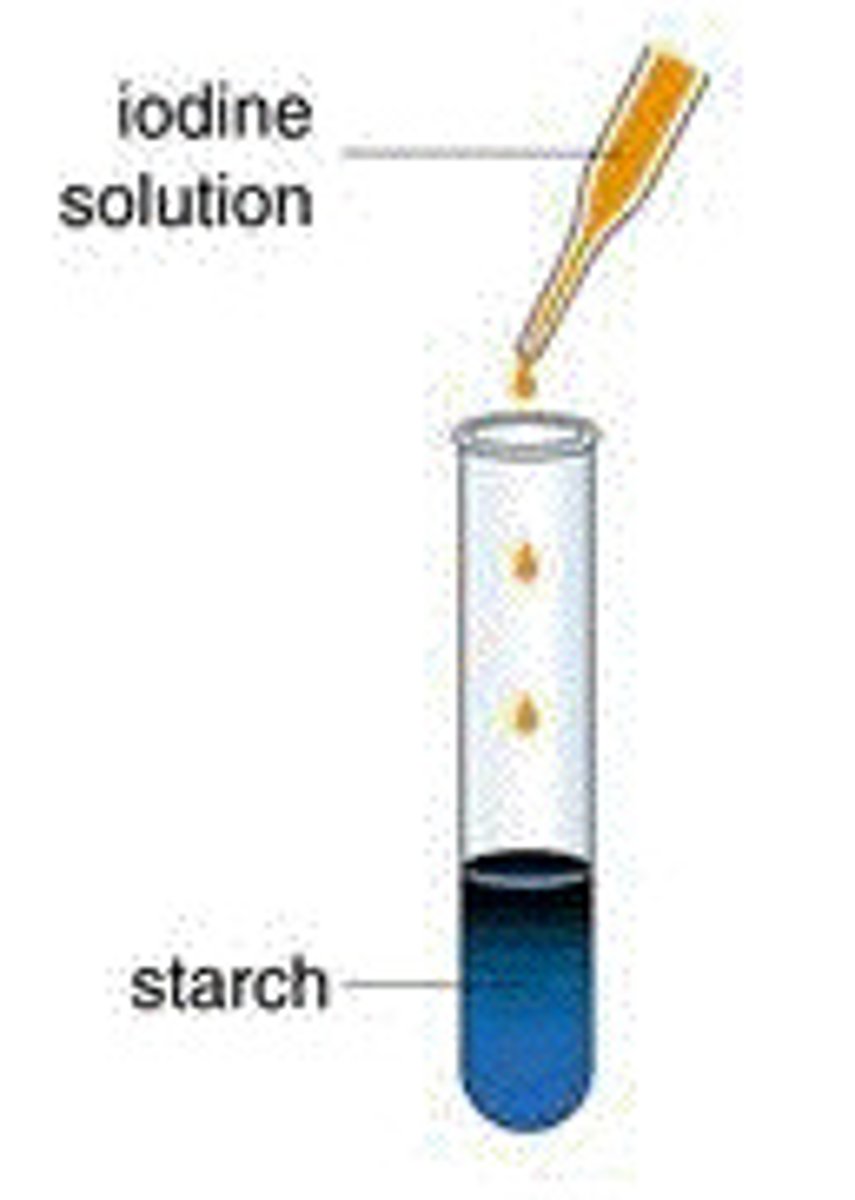
Starch test
- Iodine solution
- Yellow -> Blue-black
Lipids test
- Dissolve in ethanol and add distilled water
- White cloudy emulsion layer

How can the Benedict's test be made quantitative?
- Put excess Benedict’s reagent
- Place sample in a centrifuge to get unused Benedict’s reagent.
- Use colorimeter
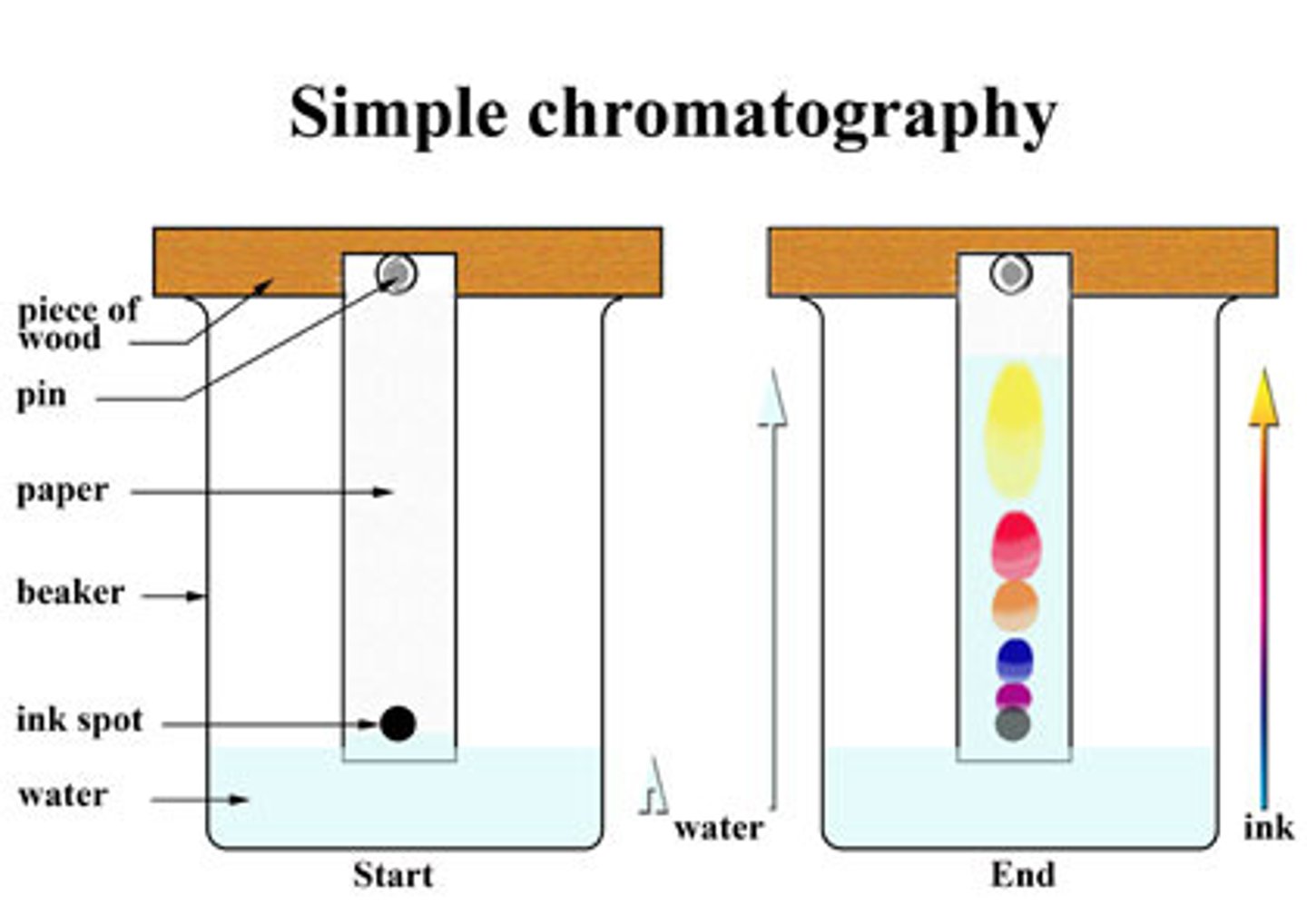
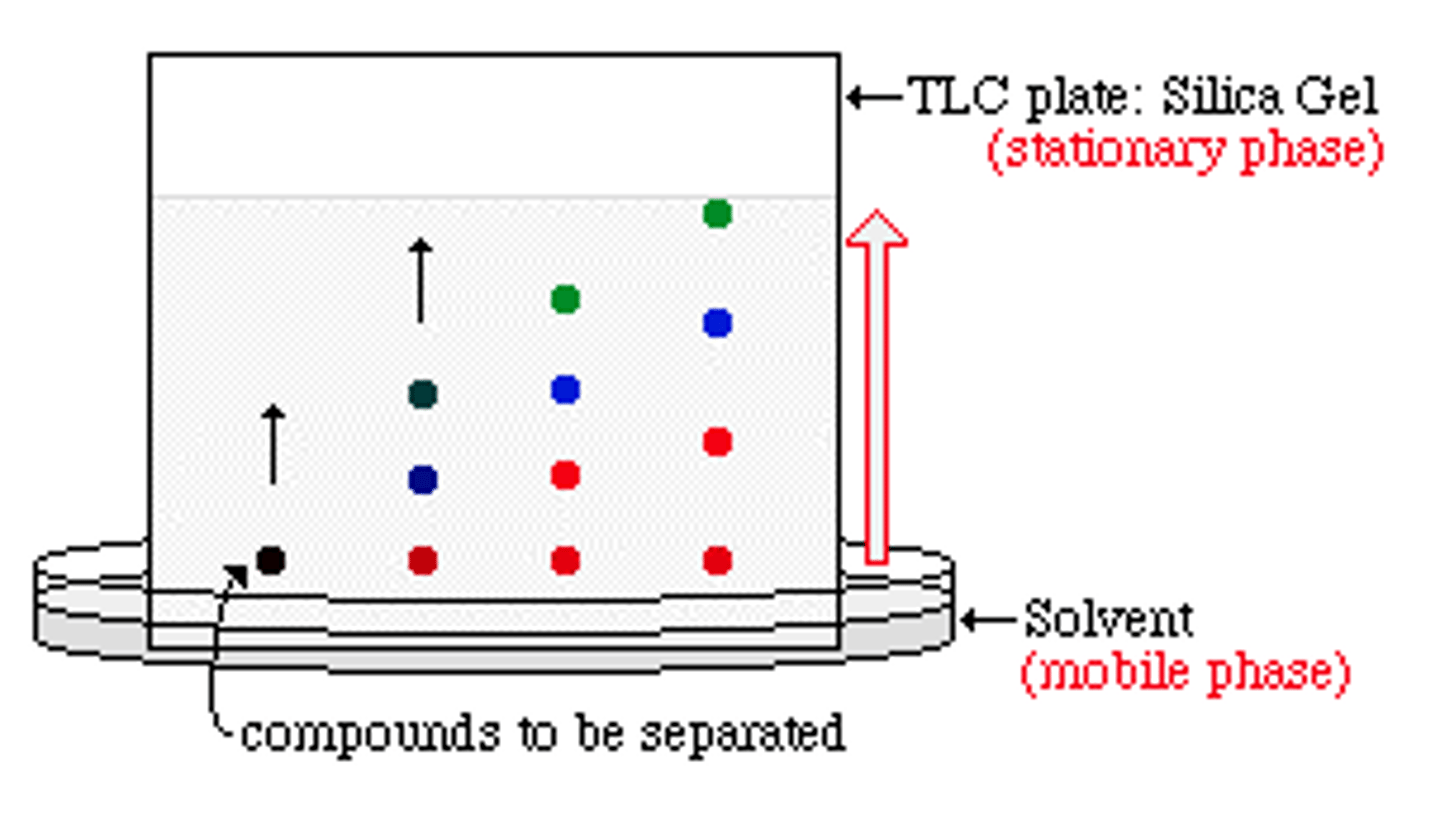
Chromatography Method
- Draw pencil line 1cm away from bottom of chromatography paper
- Place spot of substance on start line
- Place chromatography paper in beaker (using a splint and paper clip)
- Place solvent in beaker so bottom of paper just touches solvent
- Place lid over beaker
- Wait for solvent to travel 3/4 up the paper
- Mark and measure solvent front with pencil
- Dry paper and measure distance each spot travelled
- Use measurements to calculate Rf value
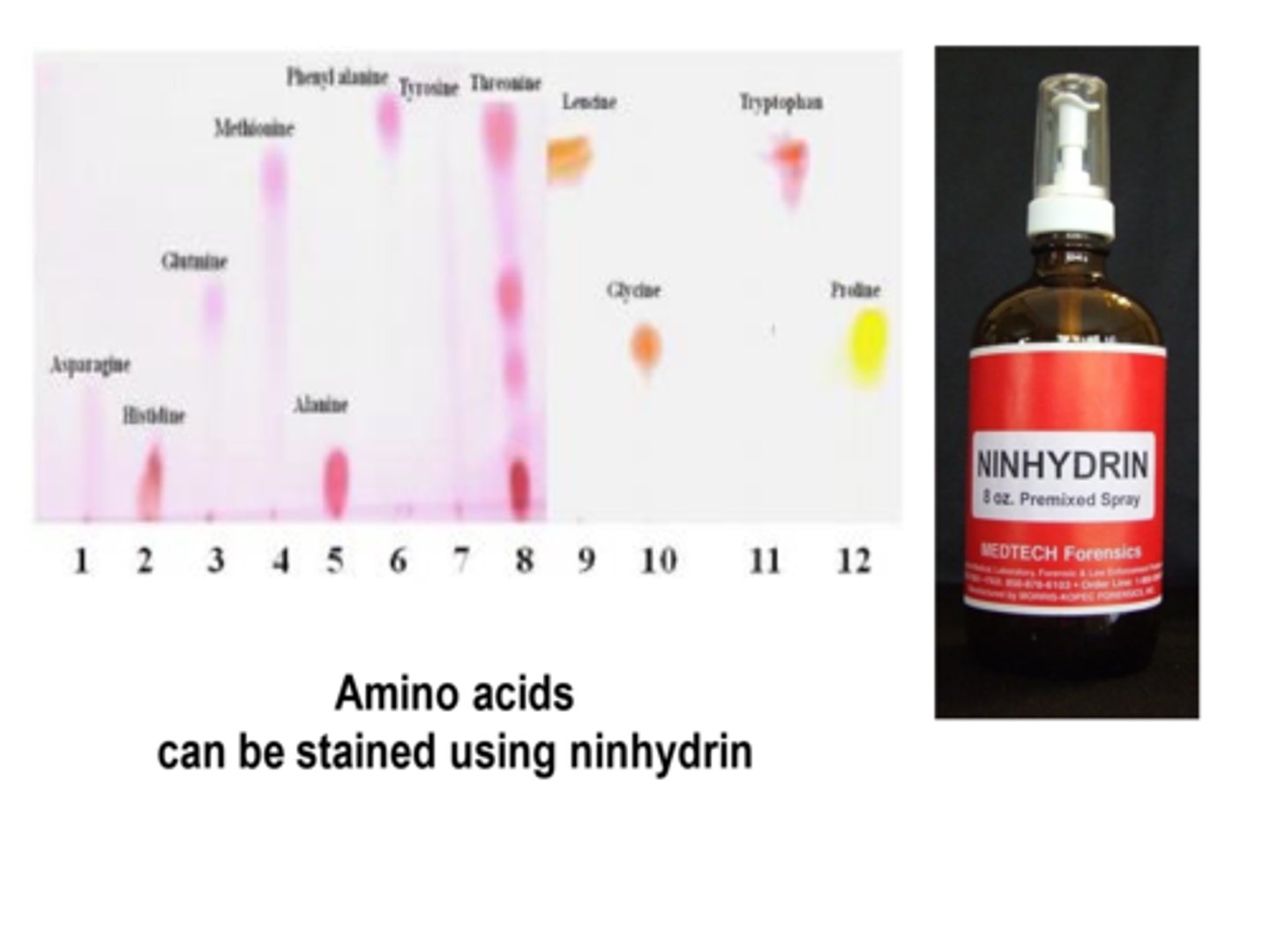
What are amino acids stained with for chromatography?
Ninhydrin
Why do substances separate in chromatography?
- Substances have different solubilties in the mobile phase
- Longer in mobile phase = Travel faster/further
How can Rf values be used to identify substances?
-When Rf value is compared to Rf values of known substances
- The higher the value, the more soluble the substance is in the solvent.
What is Thin Layer Chromatography (TLC)?
Mobile phase is still solvent, but stationary phase is a thin layer of absorbent material (eg silica gel) which is spread onto a glass sheet
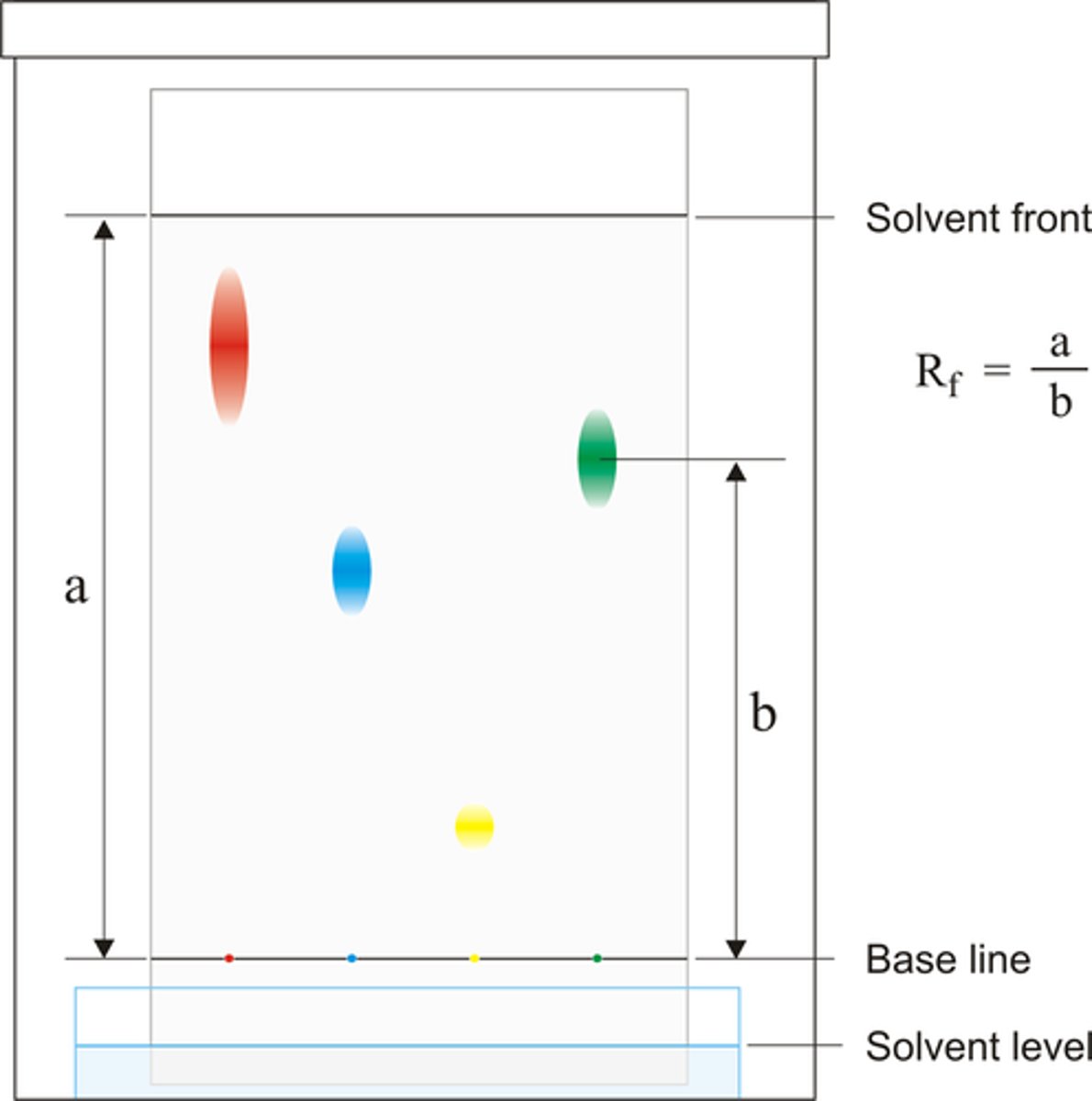
Rf value equation
Distance travelled by Substance ÷ Distance travelled by Solvent
(Rf value is always below 1)
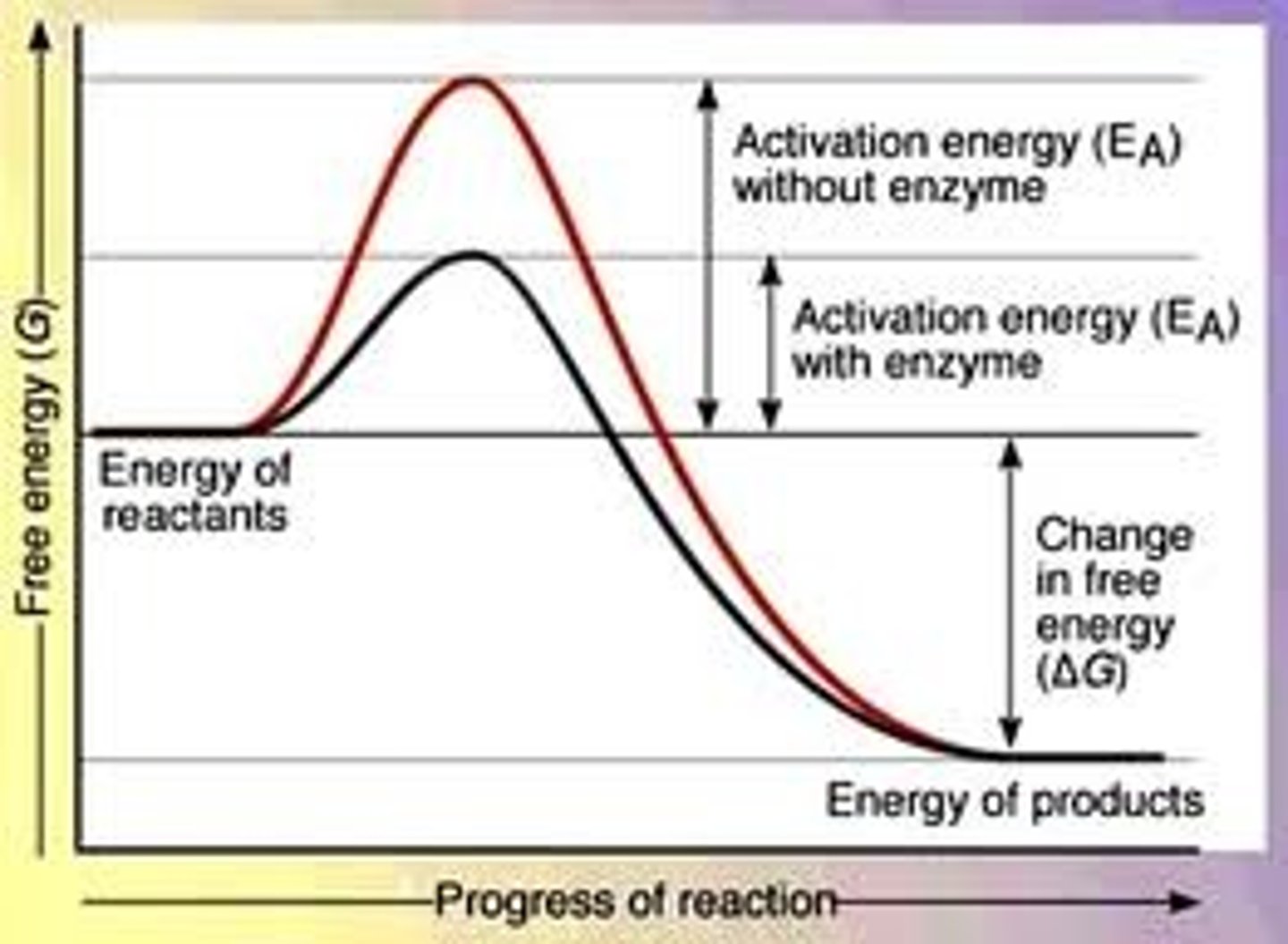
What do enzymes do?
Increases rate of reaction by lowering the activation energy
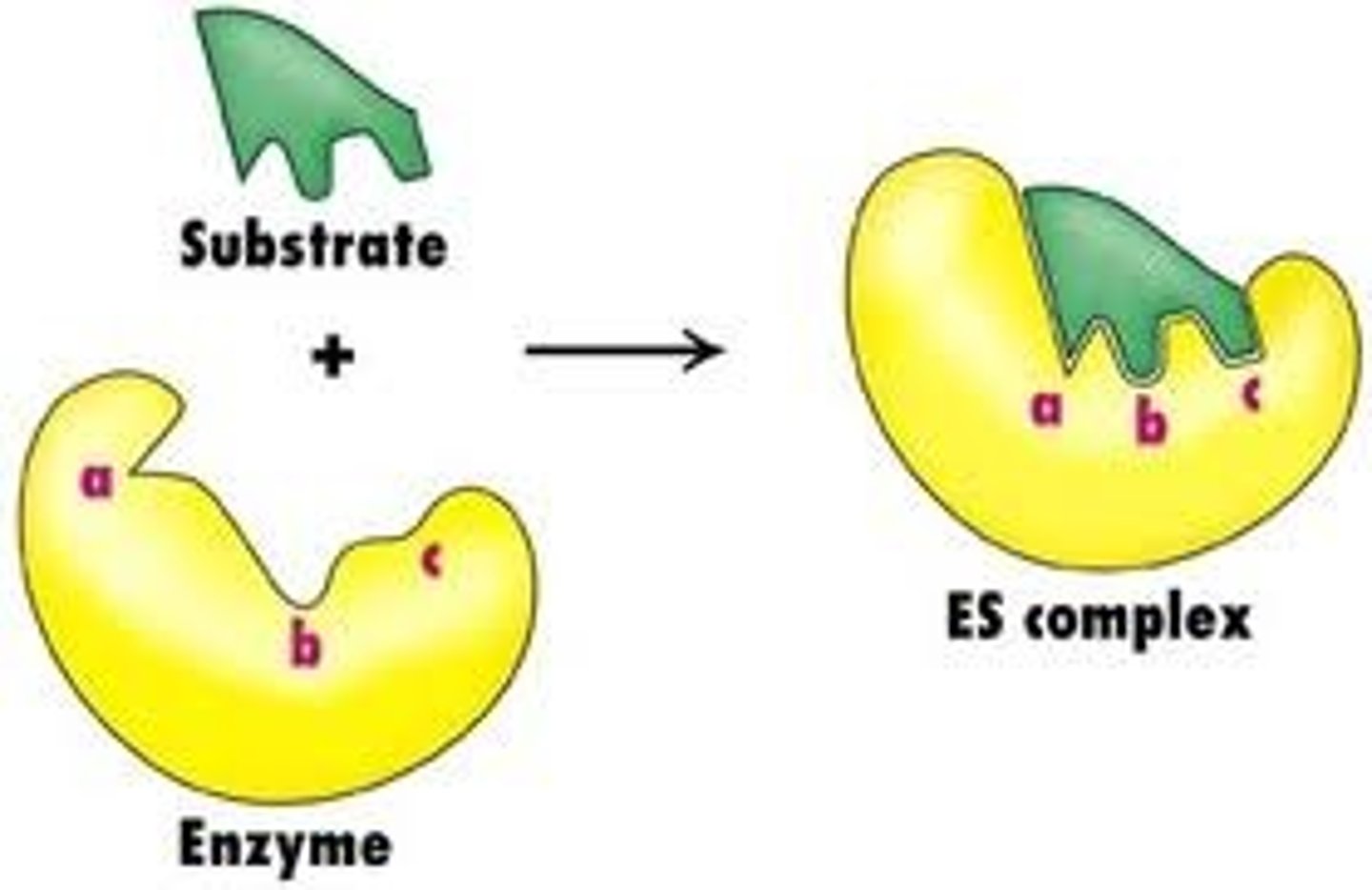
How do enzymes break down substrates?
- Substrate is complementary to the enzyme’s active site
- They combine to form enzyme–substrate complex
- This strains the bonds in the substrate, forming the enzyme-product complex
- Product leaves the active site
Examples of Extracellular and Intracellular enzymes
Extracellular (outside cell) - Amylase
Intracellular (inside cell) - Catalase
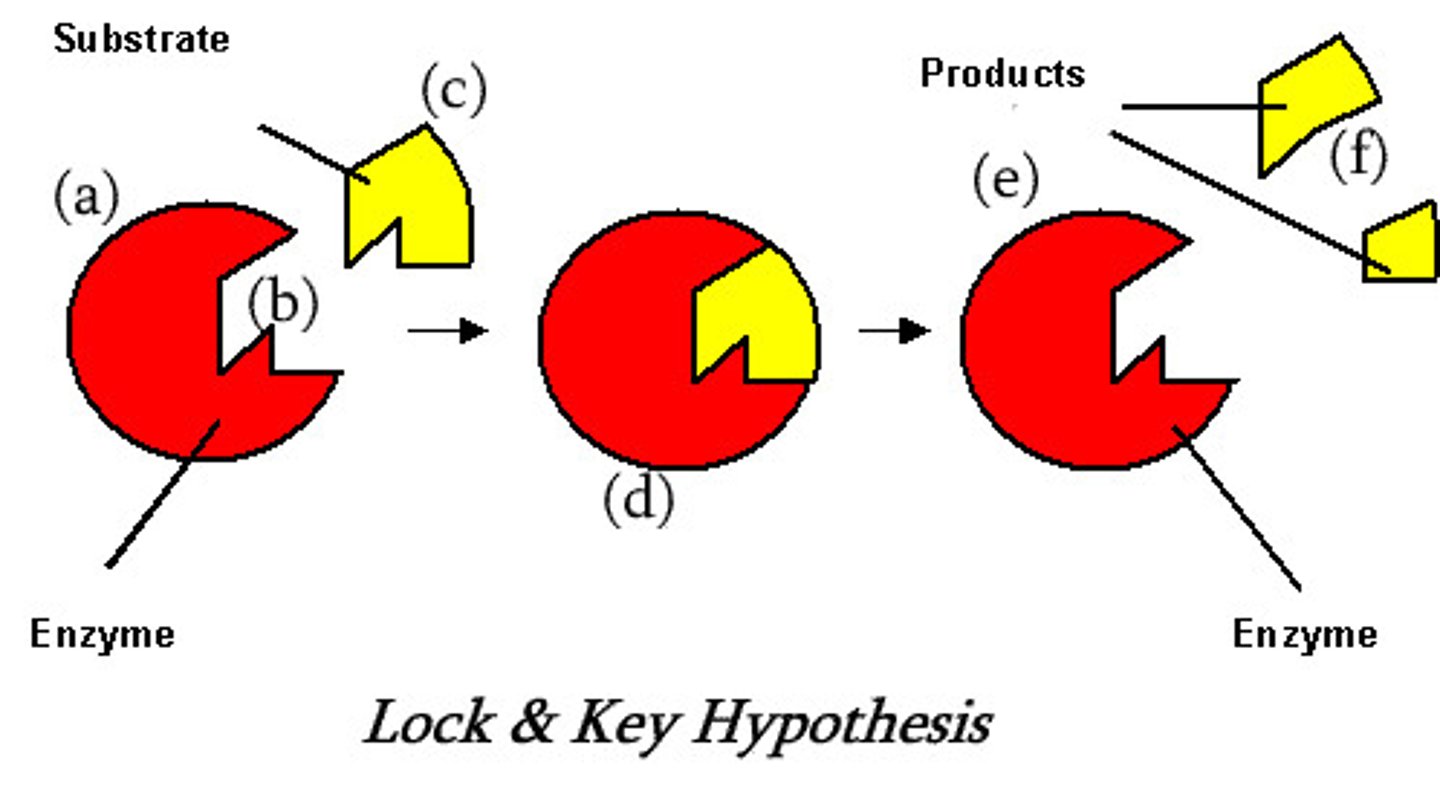
Lock and Key theory
The enzyme's active site has a specific 3d tertiary structure that is complementary to the shape of the specific substrate
Induced fit theory
- Active site of enzyme is not perfectly complementary to the shape of the substrate
- Substrate moves into the active site and bonds are strained
- Active site shape is altered to be complementary to substrate shape
- Changed active site also changes bonds in the substrate, making them easier to make/break (reducing the activation energy)
What is the temperature (Q₁₀) coefficient?
- The change in rate of reaction per 10℃ increase (usually 2)
- Q₁₀ = R2 / R1
What affects enzyme activity?
- Temperature
- pH
- Enzyme concentration
- Substrate concentration
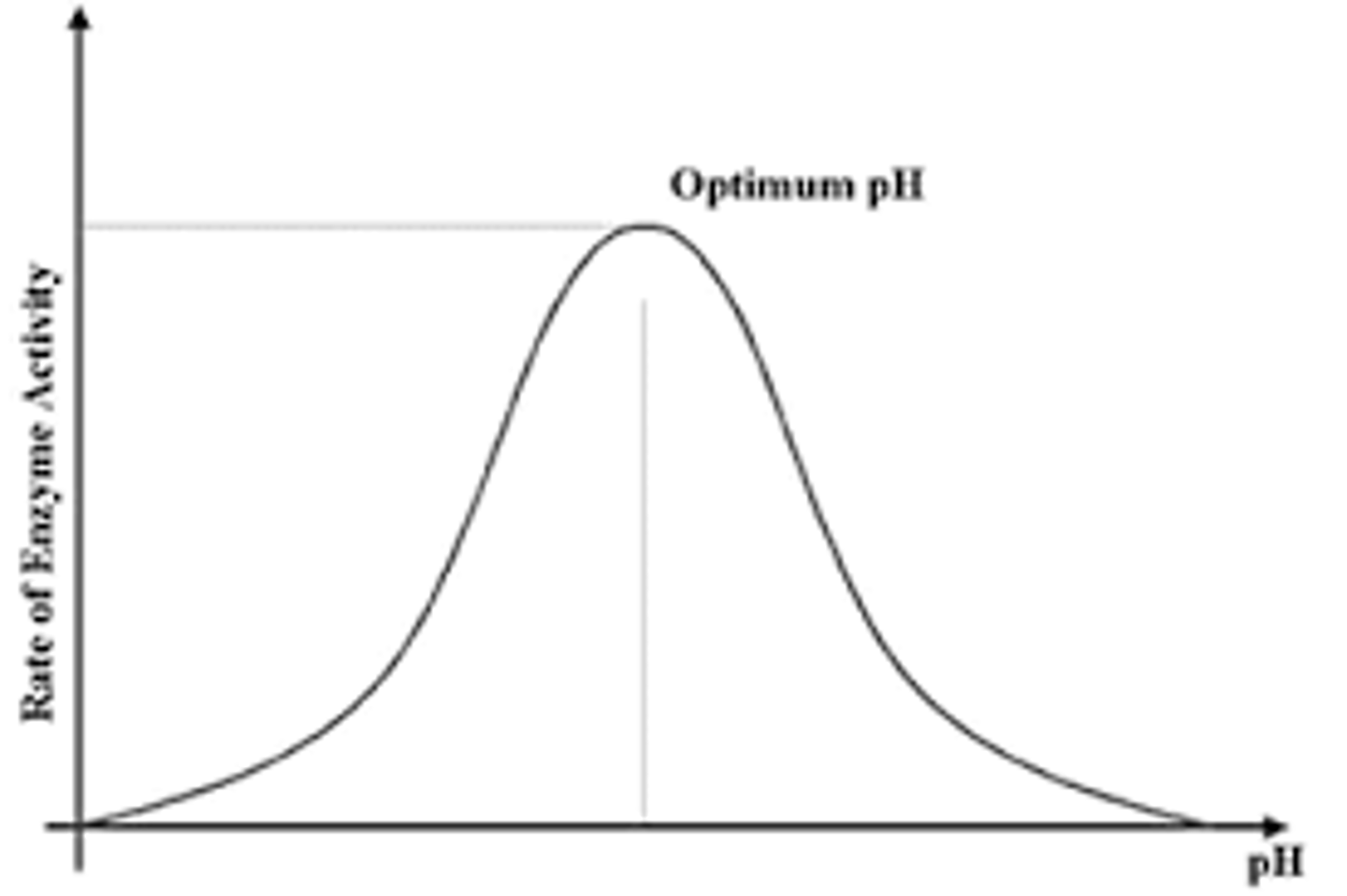
How does pH affect enzyme activity?
- Hydrogen/ionic bonds break due to high concentration of H+ ions
- Tertiary structure and active site change
- Active site is no longer complementary to substrate, so substrate doesn't fit
- Enzyme denatured
How does low temperature affect enzyme activity?
- Enzymes have less kinetic energy
- Less collisions with substrates
- Decreased rate of reaction
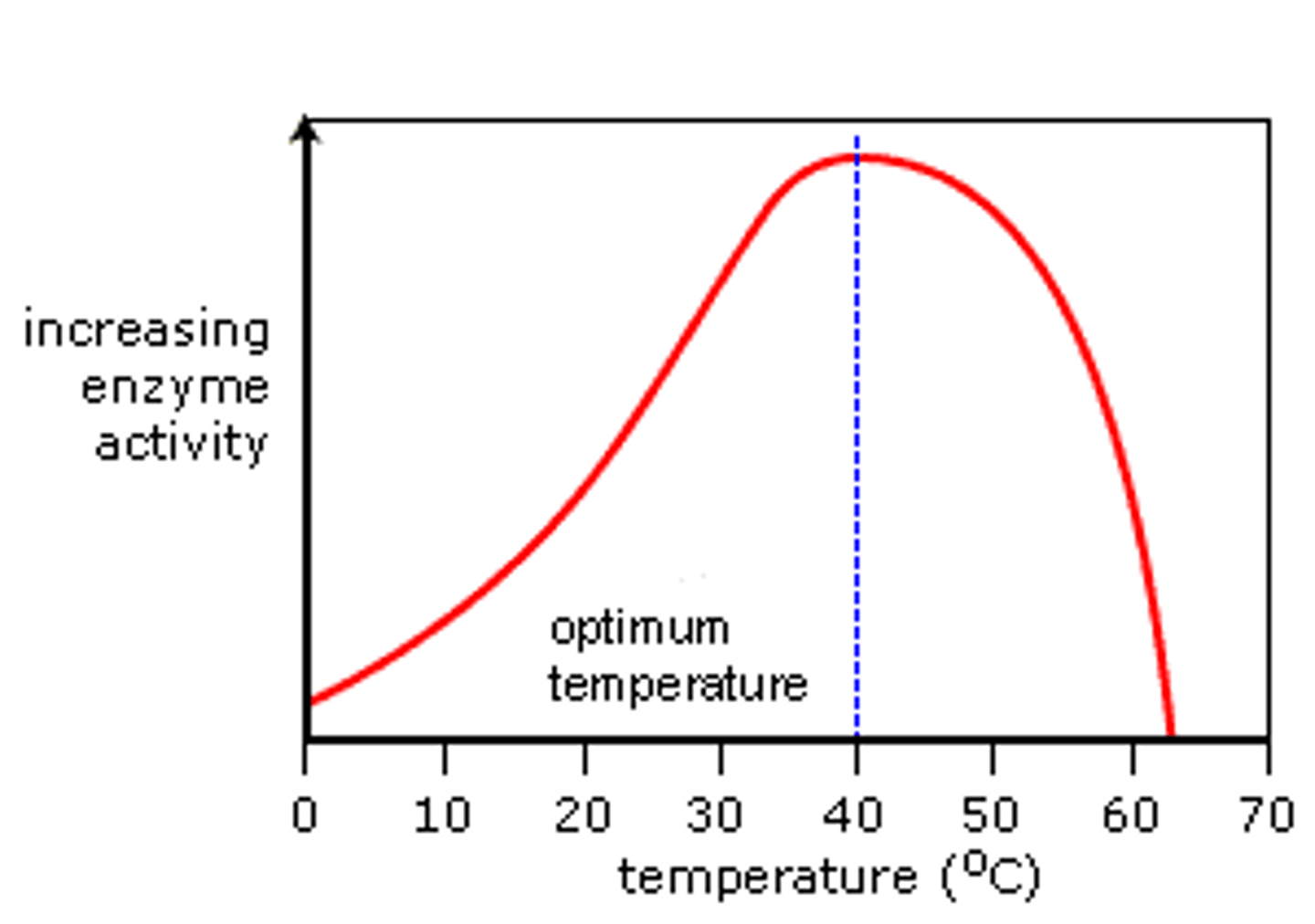
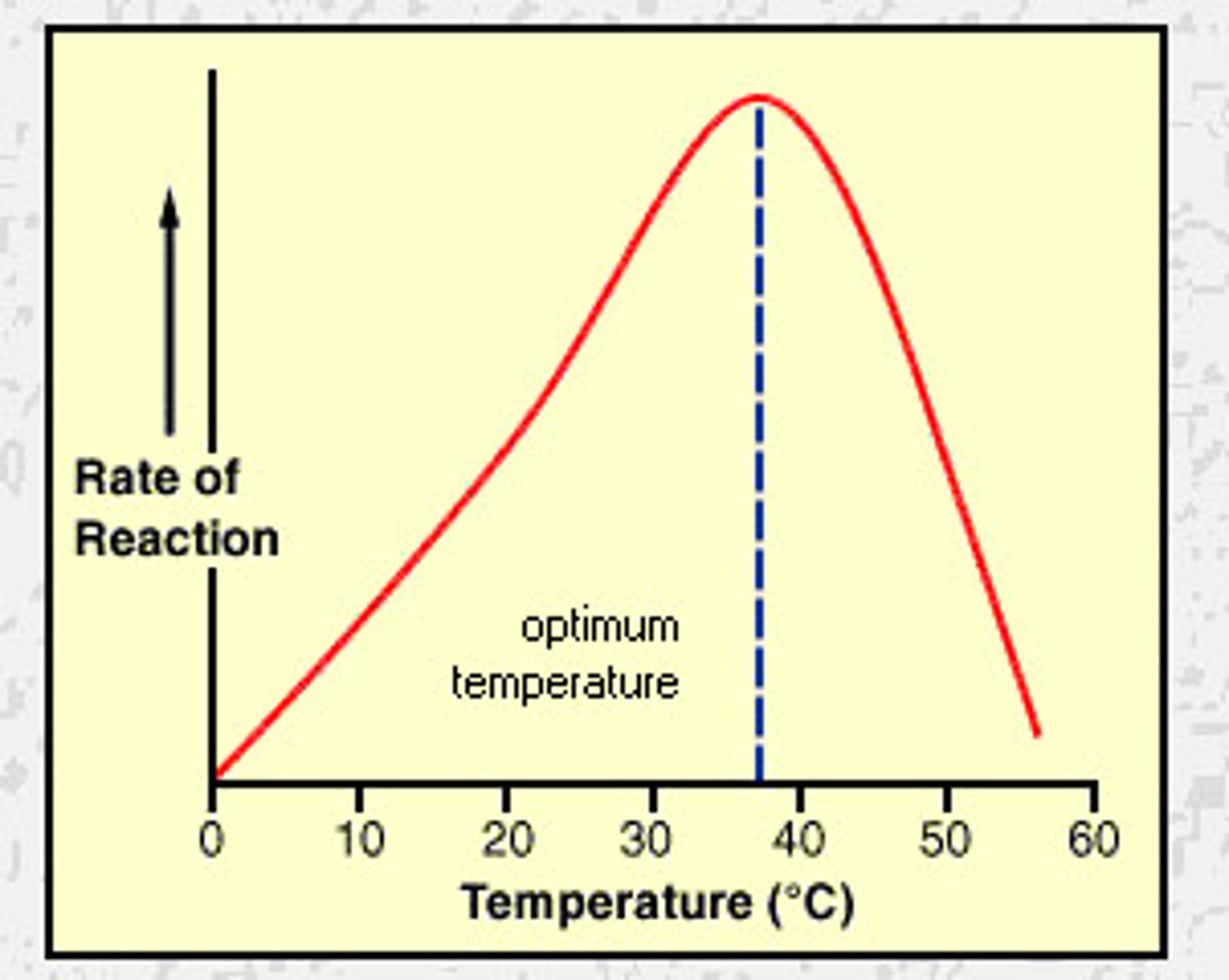
How does high temperature affect enzyme activity?
- Enzymes have high kinetic energy
- More collisions with substrates,
- More enzyme-substrate complexes
- Increased rate until optimum temp
- Past optimum, enzyme's bonds break and enzyme denatures
How does enzyme concentration affect enzyme activity?
- More enzymes means more active sites
- More collisions
- More enzyme-substrate complexes
- Increased rate of reaction (until Vmax where all substrates are occupied)
How does substrate concentration affect enzyme activity?
- More collisions between enzyme active sites and substrate
- More enzyme-substrate complexes
- Increased rate of reaction (until Vmax where all active sites are occupied)
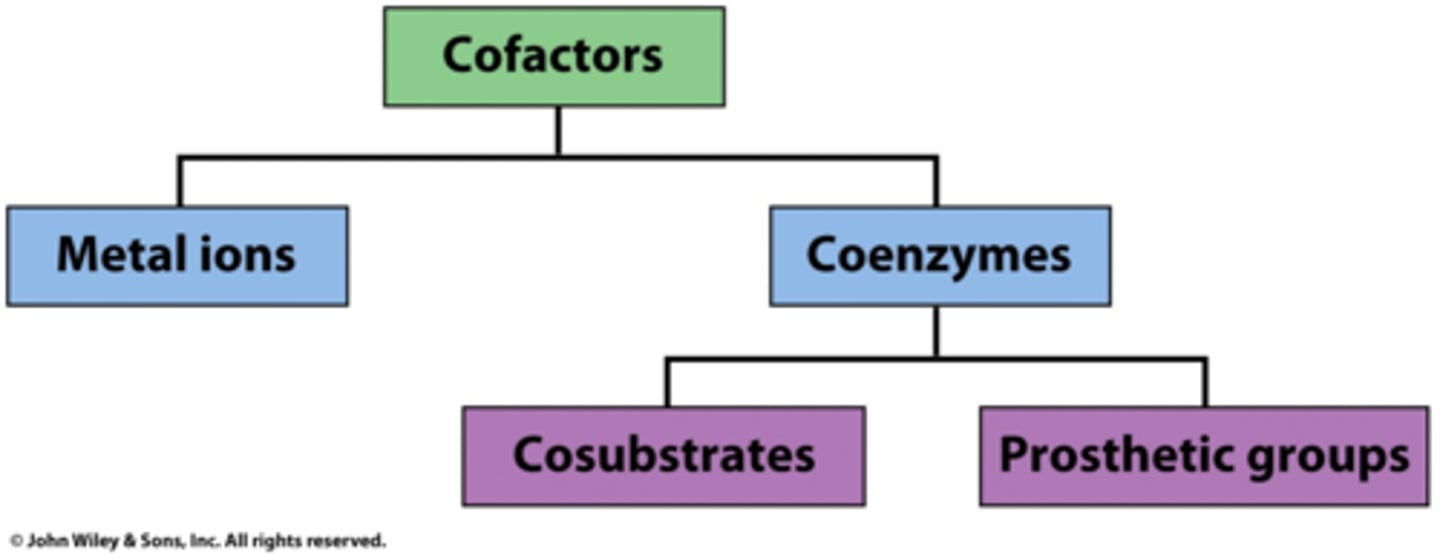
What are cofactors?
Non-protein compound required for enzyme activity to occur
Cofactors
- Inorganic ions (usually metal ions)
- Helps enzyme and substrate bind
- Temporarily bound to enzyme
- Eg: Cl– are cofactors for amylase
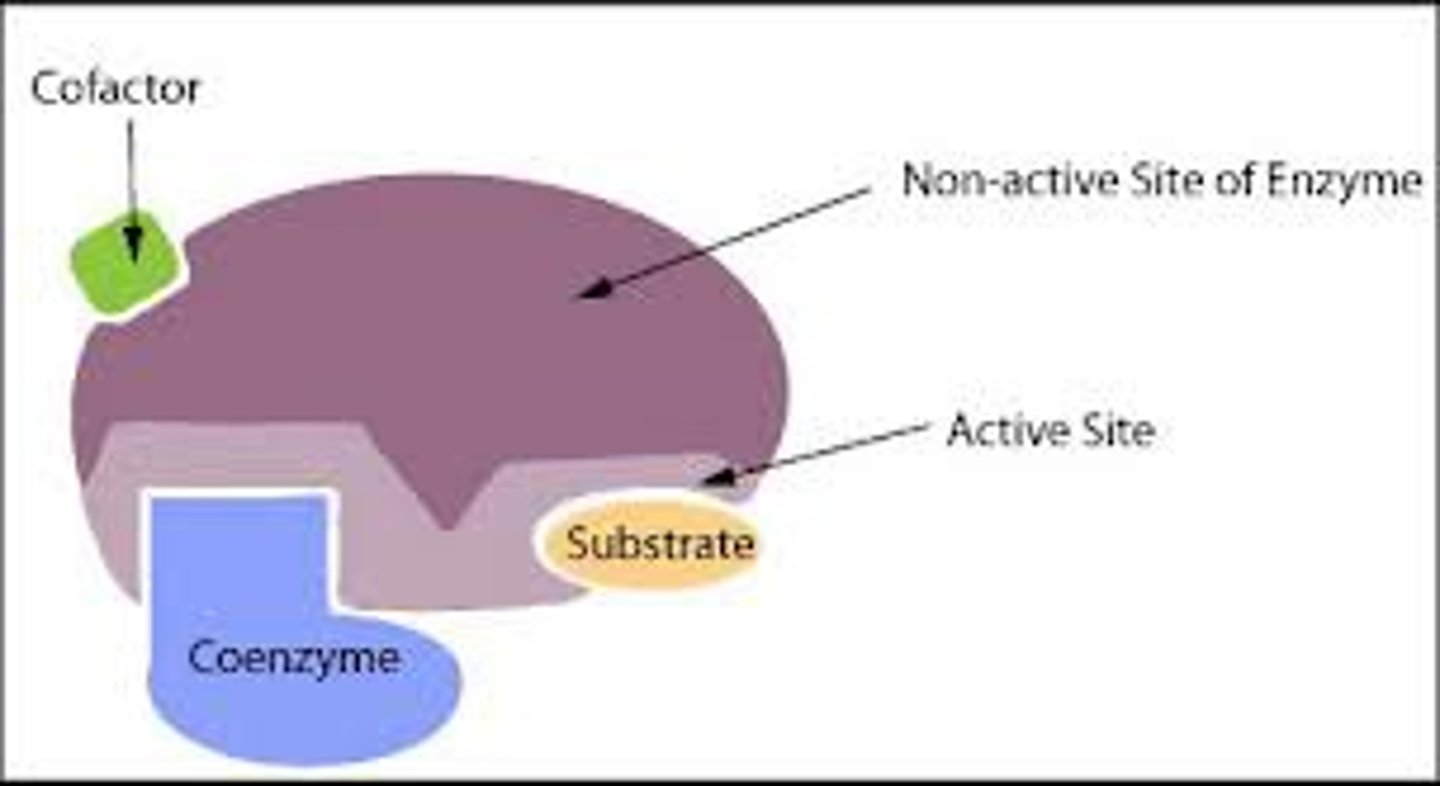
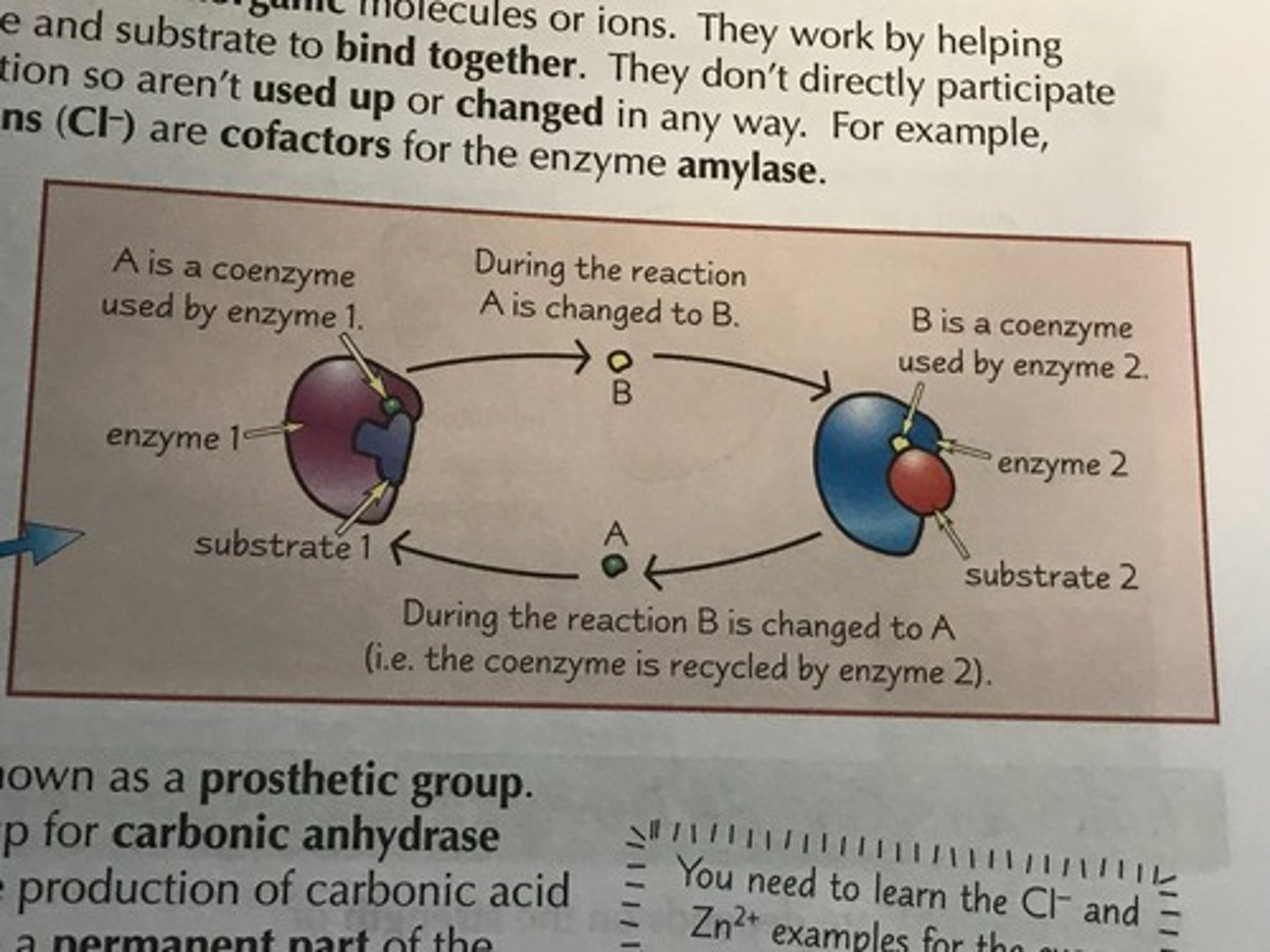
Coenzymes
- Organic substances (vitamin devired)
- Act as carriers moving chemical groups between enzymes
- Participate in reaction and changed by it
- Eg: NAD, NADP (H carriers)
Prosthetic groups
- Permenantly bound to enzyme
- Zn2+ is a prosthetic group in carbonic anhydrase
What are inhibitors?
Substances that decrease the rate of enzyme reaction
Competitive inhibitors
- Similar structure to the substrate so complementary to enzyme's active site
- Competes with substrate for the enzyme active site
- Decreases rate of reaction
- Increasing the substrate reverses the effect of competitive inhibitors by outcompeting them
