Psychopharmacology
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
56 Terms
Psychotropic medications
-Sedative-hypnotic
-Anti-anxiety
-Antidepressants
-Antipsychotics
-Dementia medications
Sedative-hypnotic and anti-anxiety drugs’ primary goal
-Relax the patient and promote “normal” sleep
-decreased anxiety
-included benzodiazepine and non-benzodiazepines
Benzodiazepines
-cause sedation
-used as “sleeping pills”
-included sedative hypnotic and anti-anxiety drugs
Benzodiazepines MOA
-Increase the effects of GABA
→GABA is inhibitory neurotransmitter that decreases excitation throuhgout CNS
-Benzodiazepines and GABA bind to a specific receptor (GABAa) in the brain
→Therapeutic effects- sedation, hypnosis, decreased anxiety
-GABAa- subunits: alpha, beta, gamma, with subsequent subdivisions
Non-benzodizepines - newer seddative hypnotics
-Barbiturates
→When used at sedative-hypnotic doses function similar to benzodiazepines to potentiate the inhibitory effects of GABA
→At higher doses may also directly reduce effects of glutamate ans also depress neuronal excitability in other areas of the brain and spinal cord
-Non benzodiazepines sedative-hypnotics
→bind preferentially to GAGAa receptor
-These ARE NOT benzodiazepines, but still bind to GAGA (alpha1 subunit) receptors in the brain
→DO NOT bind at the same place as benzodiazepines
-May produce fewer problems when discontinued
Newer sedative hypnotics
Other non-benzodiazepines
-Alcohol (ethanol) and substances with alcohol-like properties
→work through mechanisms poorly understood
→May act on protein receptors and activates GABAa receptors increasing inhibition in CNS
-Certain antihistamines, antidepressants, antipsychotics, anticonvulsants, and opioid analgesics also have sedative properties and may be used in limited situations to promote sleep
Sedative-hypnotic pharmacokinetics
-Highly lipid soluble and absorbed easily from the GI tract
-Systemic distribution with the ability to reach the CNS
-Termination by hepatic enzymes or storage in non-CNS tissues
Newer anti-anxiety drugs
-used to treat generalized anxiety disorders, possible panic disorder, OCD, and PTSD
-May decrease anxiety with less sedation, ;ess physical dependence, and addiction
-Drawbacks: slow onset, moderate efficacy
Use of antidepressants as anxiolytics
-Anxiety often occurs with depression
→patients may need both types of drugs
-Antidepressants can have direct effects- affect either serotonin or serotonin-norepinephrine balance in brain
-May alc0 have fewer side effects; less chance for addiction
sedative-hypnotic drugs: Adverse effects
-residual (hangover) effects
-retrograde amnesia
-complex behaviors (sleep walking/driving, especially with Ambienn)
sedative hypnotic and anti-anxiety drug adverse effects
-Rebound effect (insomnia, inc anxiety)
-FALLS
-Tolerance and dependence
-Benzodiazepines: possible link to Alzheimer's disease
sedative-hypnotic and anti-anxiety drugs rehabilitation concerns
-These drugs treat sxs, not the cause of the insomnia or anxiety
-Consider the trade-offs: benefits versis sedation
-There appears to be a trend toward the use of non-benzodiazepine sedative-hypnotics and axiolytics
Depression
-loss of the ability to enjoy life
-most common mental illness (characterized by feelings of sadness and despair)
-sadness that is incapacitating
-There is a neurochemical basis for depression
depression drug strategy
-increase or prolong the effect of one or more of the amine neurotransmitters
Types of antidepressants
-SSRI
-SSNRI
-Tricyclics
-MAO inhibitors
-Others
Selective serotonin reuptake inhibitors (SSRI)
-block reuptake of serotonin in the presynaptic terminal to allow serotonin to remain in the synaptic cleft and continue to exert effects longer
-if primary problem related to serotonin, may experience greater efficacy compared to other categories
selective serotonin-norepinephrine reuptake inhibitors (SSNRI)
-decrease serotonin and norepinepherine reuptake, but not dopamine
-beneficial in treating other conditions (chronic pain associated with OA, peripheral neuropathy, and fibromyalgia)
Tricyclics
-Non selective
-Affects synapses using all 3 primary amine neurotransmitters- serotonin, norepinepherine, dopamine
-Less used in favor of newer agents
MAO inhibitors
-Monoamine oxidase (MAO)- enzyme on amine synapses that removes released transmitters through the enzymatic destruction
→inhibit enzyme and more transmitter remains in synaptic cleft to exert effect
-More of a last resort drug vs a drug of choice
Other antidepressants
-These drugs are usually more complex, but are an option for some patients
→drugs that block serotonin receptors AND serotonin reuptake
→drugs that are norepinepherine-dopamine reuptake inhibitor
→drugs that may block presynaptic norepinephrine and serotonin receptors
Mechanism of antidepressant drugs
-Antidepressants prolong the effects of amine neurotransmitters by either:
→inhibiting of one or more amine neurotransmitters (SSRI; SSNRI; tricyclics; others)
→Decreasing neurotransmitter breakdown (MAO inhibitor)
Mechanism of MAOIs
-monoamine oxidase breaks down norepinephrine, serotonin, and dopamine
-when monoamine oxidase is inhibited, norepinephrine, serotonin, and dopamine are not broken down, increasing the concentration of all 3 neurotransmitters in the brain
-the transmitter is recycled and can be used over again
-20-40% of the transmitter is destroyed by the enzyme
Why might increased amine neurotransmitters decrease depression
-antidepressant drugs increase the activity of amino amine neurotransmitters
-increased neurotransmitter activity increases the brain-derived neuropathic factor (BDNF)
-BDNF stimulates growth (neurogenesis)in the hippocampus
Antidepressants: adverse effects - tricyclics
-sedation
-anticholingeric effects (dry mouth, confusion, urinary retention, constipation, tachycardia)
-Cardiovascular effects (arrhythmias and OH, especially in elderly
-seizures
-increased risk of a fatal overdose
Antidepressant adverse effects: MAOI
-CNS excitation
-increased BP (especially with other drugs/foods that cause catecholamine release)
Antidepressant adverse effects: SSRI & SSNRI
-generally better tolerated
-may increase risk of seizures
-some GI problems
other adverse effects: Serotonin syndrome
-possible with use of any antidepressant
-occurs when the CNS serotonin receptors are overstimulated
-Sxs: increased HR and BP, confusion, hallucinations, agitation, sweating, shivering, dystonia, dyskinesia, muscle pain, GI problems
-Usually reversible if addressed immediately. can be fatal if left unchecked
Antidepressants prescribed for off-label use
-common syndromes: fibromyalgia, neuropathic pain, HA, LBP, Raynaud’s phenomenon, other chronic pain syndromes
Antidepressants: rehabilitation concerns
-time lag before the beneficial effects are realized
-chance of increased depression during initial tx
-need to recognize/acknowledge mood changes
bipolar syndrome
-may be referred to as “manic-depressive” disorder
=associated with mood swings from one extreme (mania_ to another (depression)
-depressive episodes are similar to those in previous slides
-Manic episodes: euphoria, hyperactivity, talkativeness
bipolar syndrome - Lithium
-Antimanic
-Prevents manic episodes
-considered the best tx for bipolar syndrome
-MOA unclear: may stabilize neurons, may be neuroprotective
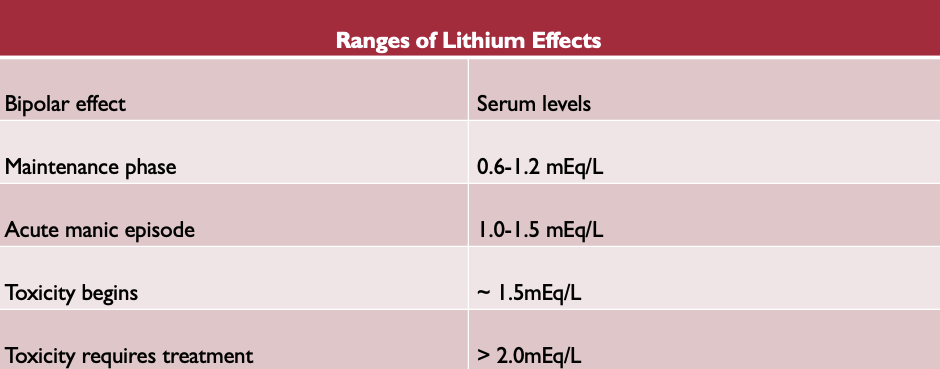
Lithium ion itself and pharmacokinetics
-is in the same category as sodium
-is an element not degraded in the body- not metabolized
-eliminated intact by the kidneys
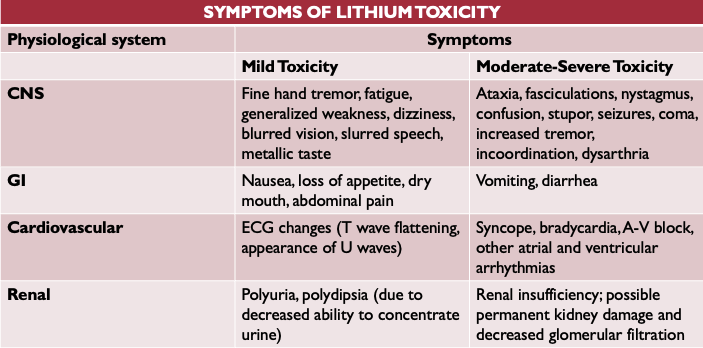
-can accumulate rapidly
-Lithium toxicity
Sxs of lithium toxicity
Ranges of lithium effects
other meds used to treat bipolar syndrome
-Antiseizure drugs
-antipsychotics
Bipolar disorder rehab concerns
-Be alert for behavior changes that may indicate that the drug is reaching toxic levels
-may need to alert the physician/healthcare practitioner
Antipsychotic medications - psychosis definition and cause
-psychosis: more severe forms of mental illness
-Cause: increased dopamine activity in specific CNS pathwaysan
antipsychotic meds - other neurotransmitters that may be involved (other than dopamine)
-5-HT
-Glutamate
-Acetylcholine
Antipsychotics MOA
-BLOCK CNS dopamine receptors, especially D2 receptors in mesolimbic pathways
-D2 antagonist blocks enough of the receptors so that when the dopamine is released, it doesn’t over-stimulate the post-synaptic terminal
Antipsychotics traditional vs “atypical”
-Traditional: original antipsychotic meds
-” Atypical”: newer than traditional and may be preferred because of fewer/milder side-effects
Traditional antipsychotics
-tend to bind to several types of CNS dopamine receptors, including those that influence motor function in basal ganglia
-less predictable
-more patient-to-patient variability
-side effects and potential for long-term implicationsA
Atypical antipsychotics
-weak blockers of dopamine type 2 (D2) receptors
-Strong blockers of specific serotonin receptors (5-HT2 receptors)
-Clinical significance
→better at treating psychosis ?
→fewer, less serious side effects
-Less incidence of relapse due to improved tolerance and patient compliance
Antipsychotic adverse effects
-traditional agents: OH, sedation, anticholinergic effects
-Atypical agents: weight gain; disturbed lipid/glucose metabolism
-Primary concern with all antipsychotics: extrapyramidal (motor) side-effects
Extrapyramidal side-effects
-tardive dyskinesia
-pseudoparkinsonism
-akathisis
-other dystopias, dyskinesias
extrapyramidal effects - tardive dyskinesia
-prevalence: 25% of patients on long-term traditional antipsychotics
-cause: denervation super-sensitivity?
-Risk factors: advanced age; genetic predisposition; affective mood disorders; diabetes; hx of alcohol abuse (>6 months of continuous use)
-best tx: early recognition and change in type or dose of the antipsychotic drug
antipsychotics - Neuroleptic malignant syndrome (NMS)
-can occur with all antipsychotics
-Sxs: catatonia, rigidity, tremors, fever
-Increased risk if the dose is high, the patient is agitated, or has impaired mental function
-Can be fatal: critical need to detect NMS early and discontinue the drug
-REFER IMMEDIATELY
antipsychotic - Non-motor effects
-Metabolic effects: substantial weight gain; increased plasma lipids; DM
-Risk varies with drug
Antipsychotics rehab concerns
-must consider the options: benefits versus sedation
-be alert for OH
-recognize extrapyramidal side-effects
Dementia definition
-degenerative changes in the neuronal structure and function
-irreversible dementia: alzheimers disease
goals of drug therapy for dementia
-improve cognitive and intellectual function
→neuronal changes lead to decreased ACh activity in the brain
→cholinergic stimulants: increase ACh activity either directly or indirectly
-Improve/modify behavior
Indirect cholinergic stimulants: effect in dementia
-drug inhibits cholinesterase enzyme
-ACh breakdown is inhibited
=ACh activity/effects are prolonged
cholinergic stimulants - indications and efficacy
-Indications: improve cognition; behavioral function
-Efficacy: help patients retain more cognitive/intellectual function in the early stages of AD
Memantine (Namenda)
-blocks NMDA-glutamate receptors in the brain
→glutamate: excitatory amino acid, important in memory and learning
-glutamate activity disrupted in AD: drug therapy normalized glutamate influence
-provides another strategy to slow AD progression; sustain memory, and intellect
FDA approved monoclonal antibody drug AD that was eventually discontinued
-Aducanumab (Aduhelm)
→first to target fundamental pathophysiology of disease (amyloid plaques and neurofibrillary tangles)D
Drugs used to improve or modify behavior
-antidepressants
-antianxiety drugs
-antipsychotics
Behavior modification in AD and rehab implications
-government regulations instituted to curb the use of antipsychotics
-more emphasis on using sx-specific meds
-consider non-pharm interventions
-Rehab implications: following commands, participation, VCing, impact on safety