Reagents in Orgo for AlkAnes
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
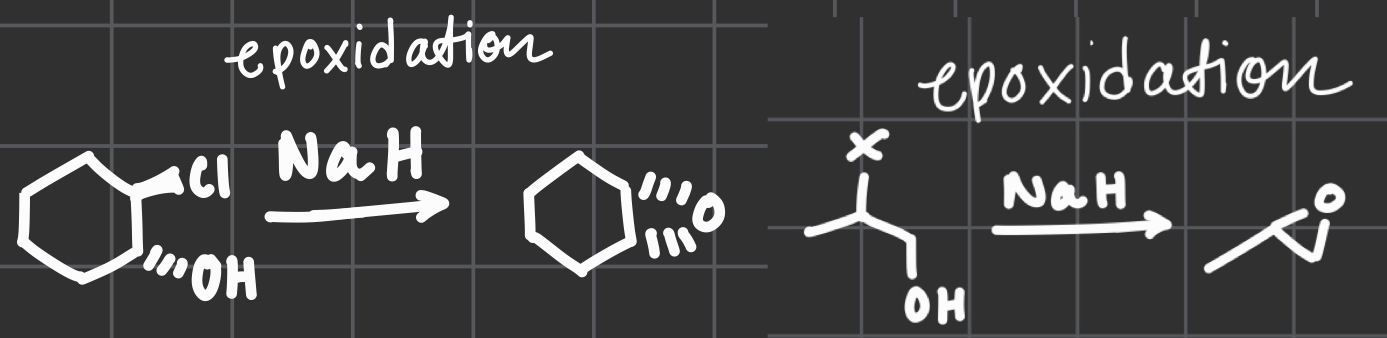
NaH
OR
NaOH
(molecule has an OH group)
Epoxidation via deprotonation of OH
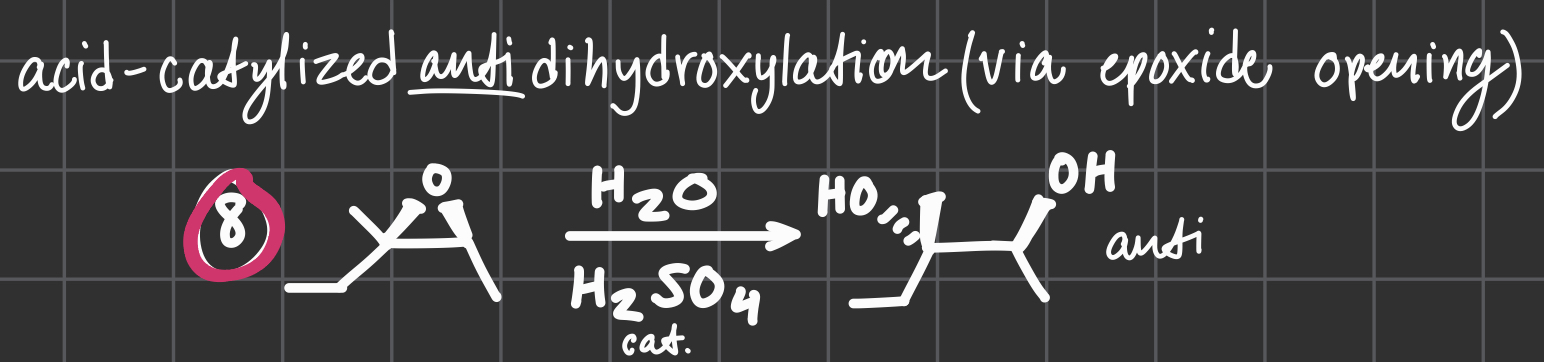
H2O
H2SO4
ACID-catalyzed anti dihydroxylation via opoxide opening
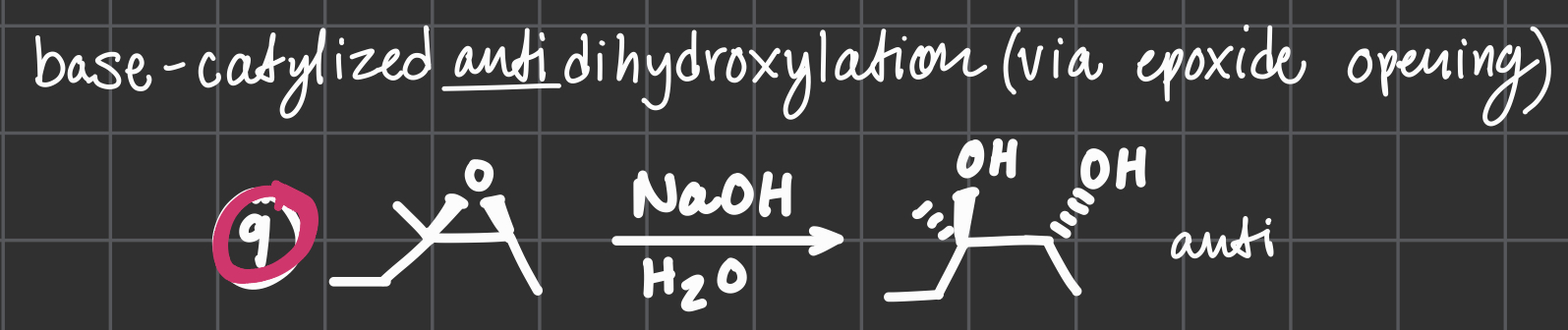
NaOH
H2O
BASE-catalyzed anti dihydroxylation via opoxide opening
HBr
Hydrohalogenation but specifically hydrobromination
(markovnikov)
NaIO4
H2O
Oxidative diol cleavage
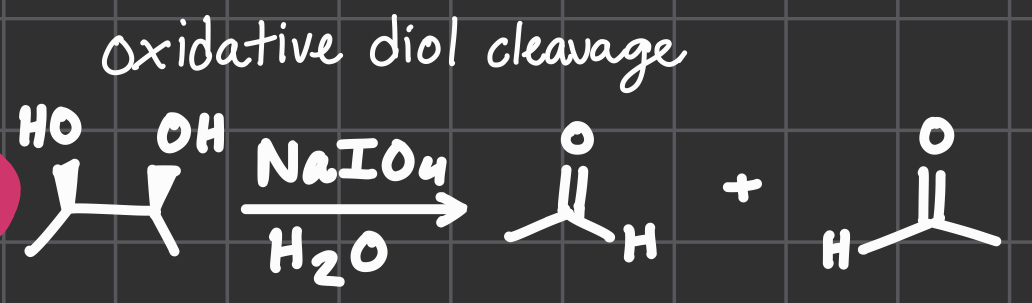
H2O2
Idk what this one called by it looks like it makes an H an OH??
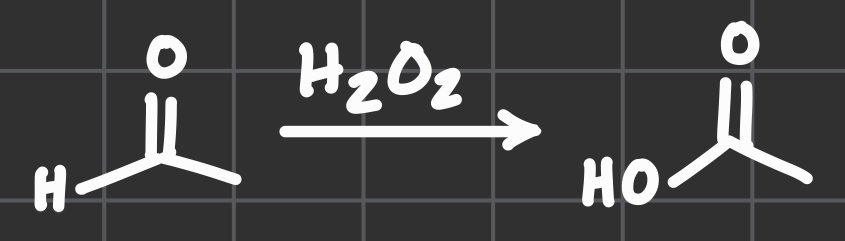
PBr3
Replaces alcohol group with Br
Swaps stereochem
OH acts as a LG

SOCl2
Pyridine
Replaces alcohol group with Cl
Swaps stereochem
OH acts as a LG
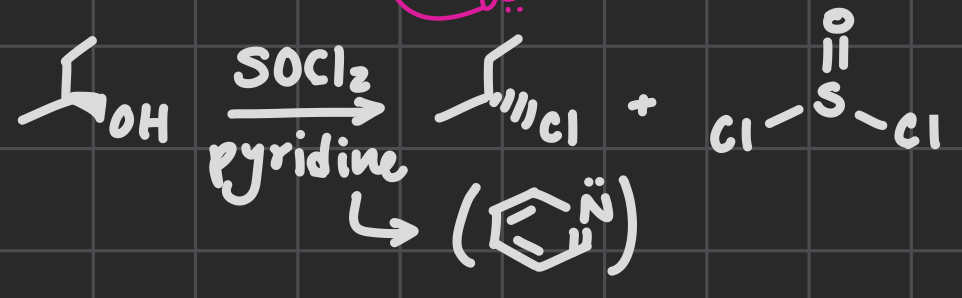
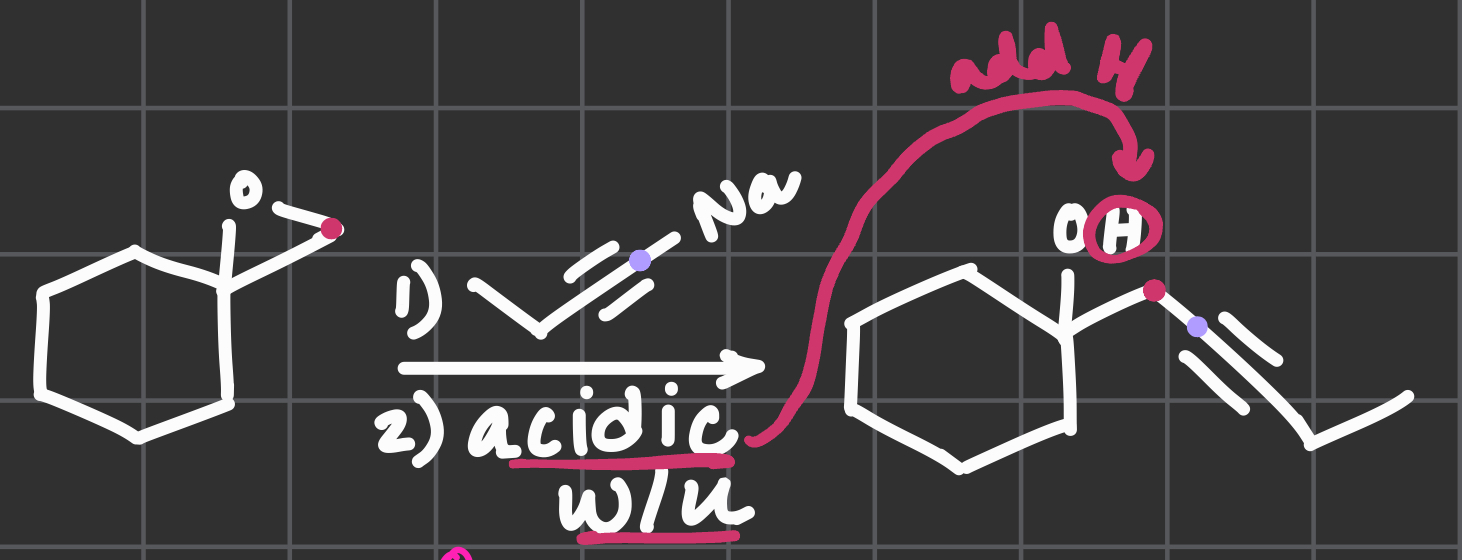
1) Na chain??
2) acidic w/up
Na is kicked out and this happens
MgØ
Grignard’s reagent
1) CO2
2) acidic workup
1) bonds with MgX
2) hydrogenates the O-
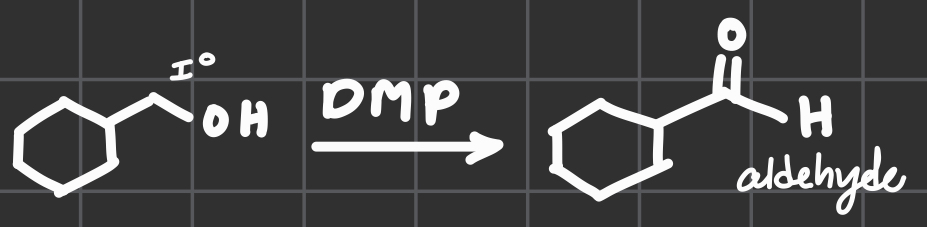
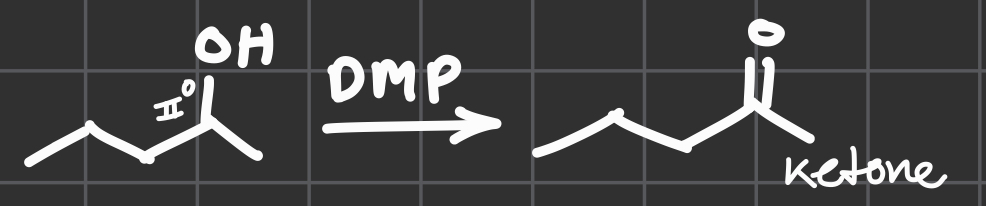
DMP
w/OH connected to I°
Get an aldehyde
DMP
w/OH connected to III°
Get a ketone
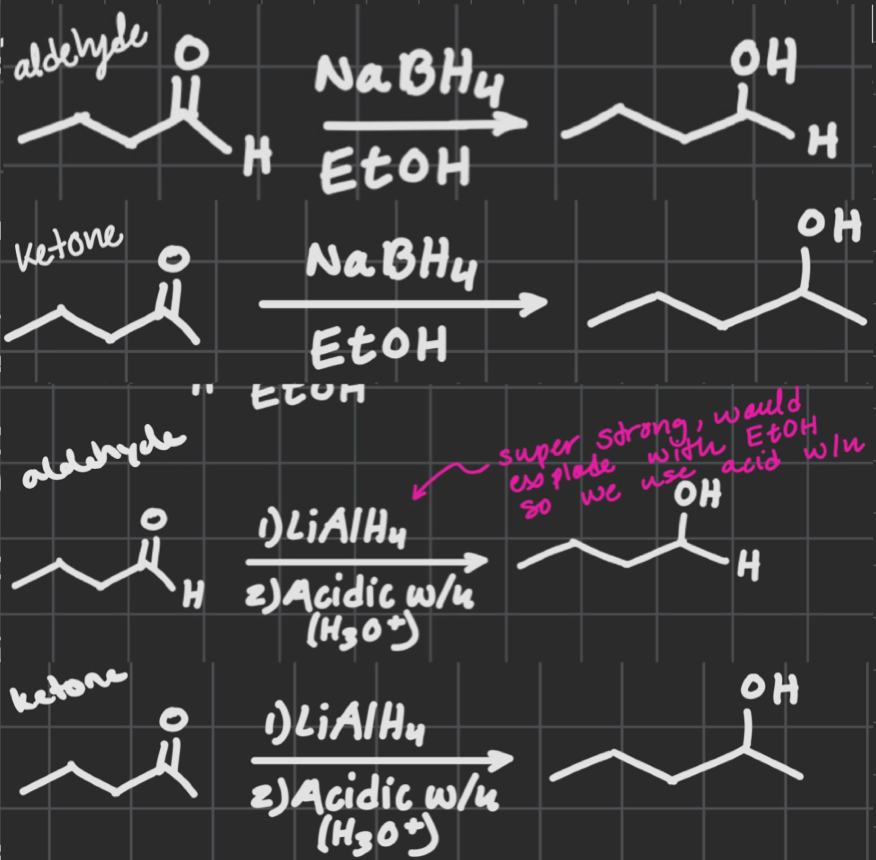
NaBH4
EtOH
OR
1) LiAlH4
2) acidic w/up
Replaces ketone w/alcohol group
NBS
HBr, Δ
Adds a Br somewhere??
TsCl
Pyridine
Replaces OH with OTS