242 Lab 3: Three step synthesis
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
what was lab 3?
Step 1: oxidation of benzoin to benzil
Step 2: tetraphenylcyclopentadienone synthesized from benzil & 1,3-diphenylacetone
Step 3: 1,2,3,4-tetraphenylnaphthalene synthesized from anthranilic acid & tetraphenylcyclopentadienone
what is efficient about this synthesis?
building several C-C bonds at a time to build up a more complex structure
what was green about this lab?
using a catalytic copper-acetic acid/ammonium nitrate system to accomplish the oxidation of the alcohol (using catalytic Cu(II), stoichiometric NH4NO3), better than chromium reagents
procedure for step 1
in rb, add benzoin, ammonium nitrate, cupric acetate monohydrate, aq acetic acid, attach H2O condenser & reflux 1 hr, take TLC @ 30 & 60 min mark, cool mixture, pour into beaker w/ DI H2O, cool in ice bath until crystals, vacuum filter, prepare KOH for step 2 by dissolving KOH in ethanol
why do we wash the crude product of step 1 with DI H2O after vacuum filtering?
to remove the nitric acid
what is a full recrystallization?
dissolving product in minimal hot solvent, then cooling to allow the pure crystals to come out, leaving the impurities behind in the solvent
what are the 3 recrystllization options after step 1?
1) carry out entire recrystallization on day 1
2) dissolve crude in hot solvent and store overnight for crystal formation
3) NOT PREFERRED, do entire recrystallization on day 2
procedure for step 2
mix benzil, 1,3-diphenylacetone, & ethanol in 10 ml rb w/ stir bar, attach reflux condenser, heat until dissolved, slowly add KOH soln (soln should turn deep purple), reflux 15 min. cool mixture in ice bath, collect precipitate & wash w/ ethanol & dry on funnel
what molecule is generated and used during the reaction in step 3?
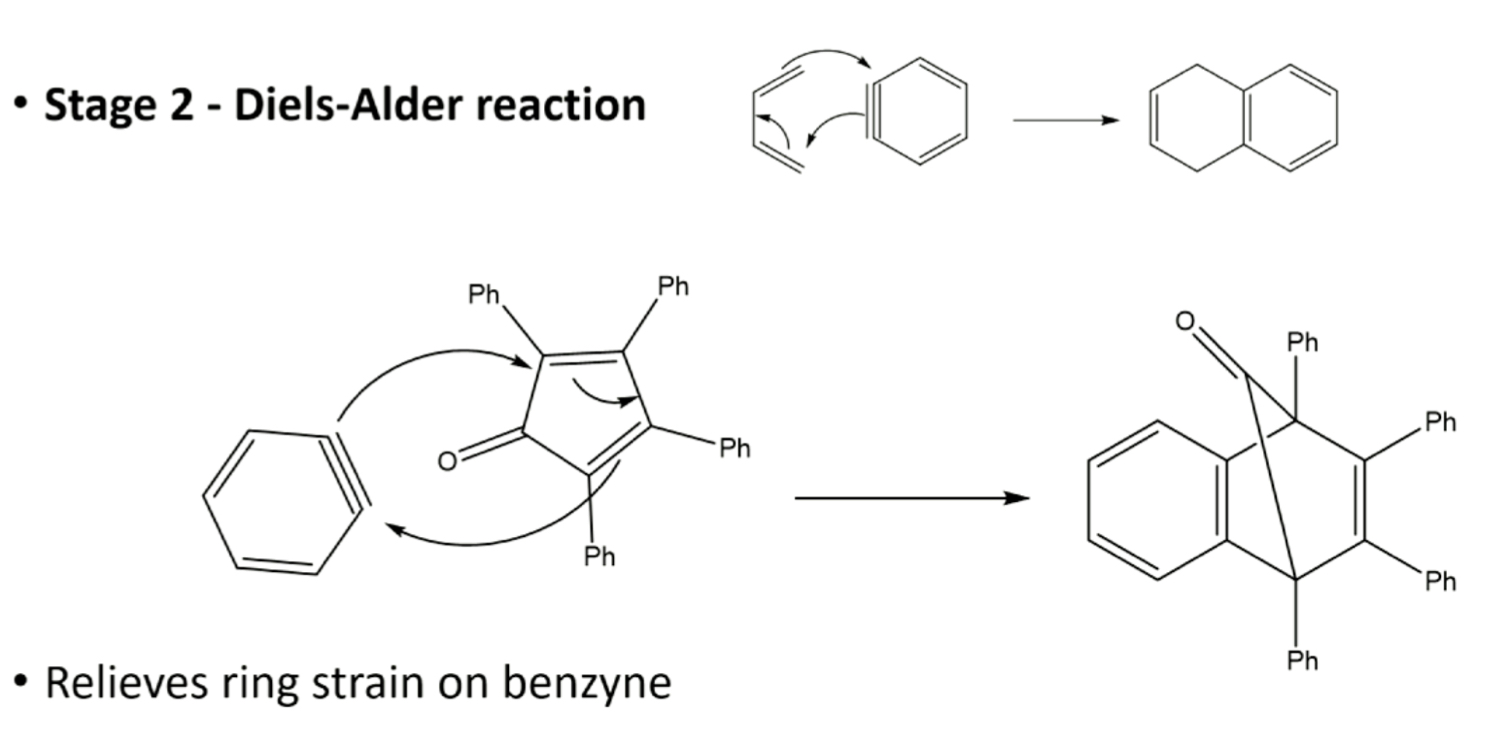
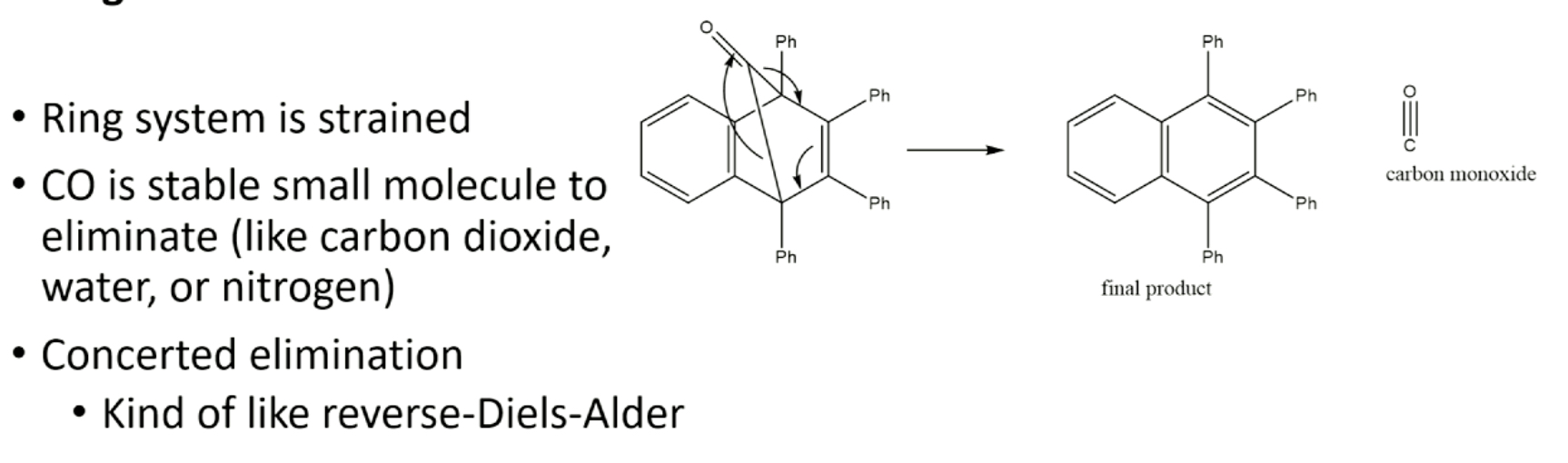
benzyne is highly reactive molecule generated in situ using anthranilic acid & isopentyl nitrite, diels alder rxn makes unstable intermediate, which after loss of CO2, gives desired product
safety information
isopentyl nitrite is vasodilator (lowers bp)
procedure for step 3
dissolve tetraphenylcyclopentadienone & anthranilic acid in DME in 5 ml conical vial with H2O condenser for reflux, reflux mixture & add isopentyl nitrite drop-wise, stir rxn until deep purple → yellow-orange, if no color change after 15 min, add 2 drops isopentyl nitrite every 2 min until color change (no more than 6 drops), precipitate out product by adding DI H2O and 2 ml methanol, cool in ice bath, filter & rinse product
why should you add the isopentyl nitrite solution dropwise through the top of the reflux condenser?
to prevent any hazardous & volatile liquids from escaping
why are we not recrystallizing the final product?
due to the insoluble nature of the final product, it is too difficult in the time given
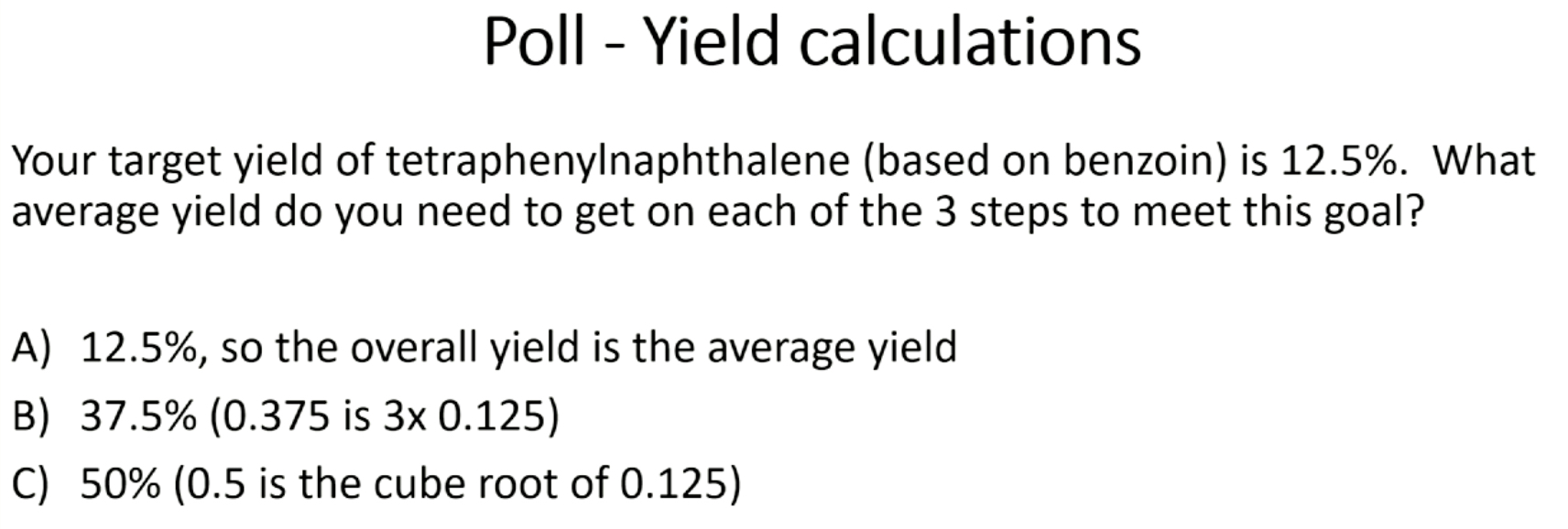
C

B & F

A & B (need both)

C
Reaction for step 1 (reactants, reagents, products)
Benzoin reacting with catalytic Cu(II) & NH4NO3 to produce benzil
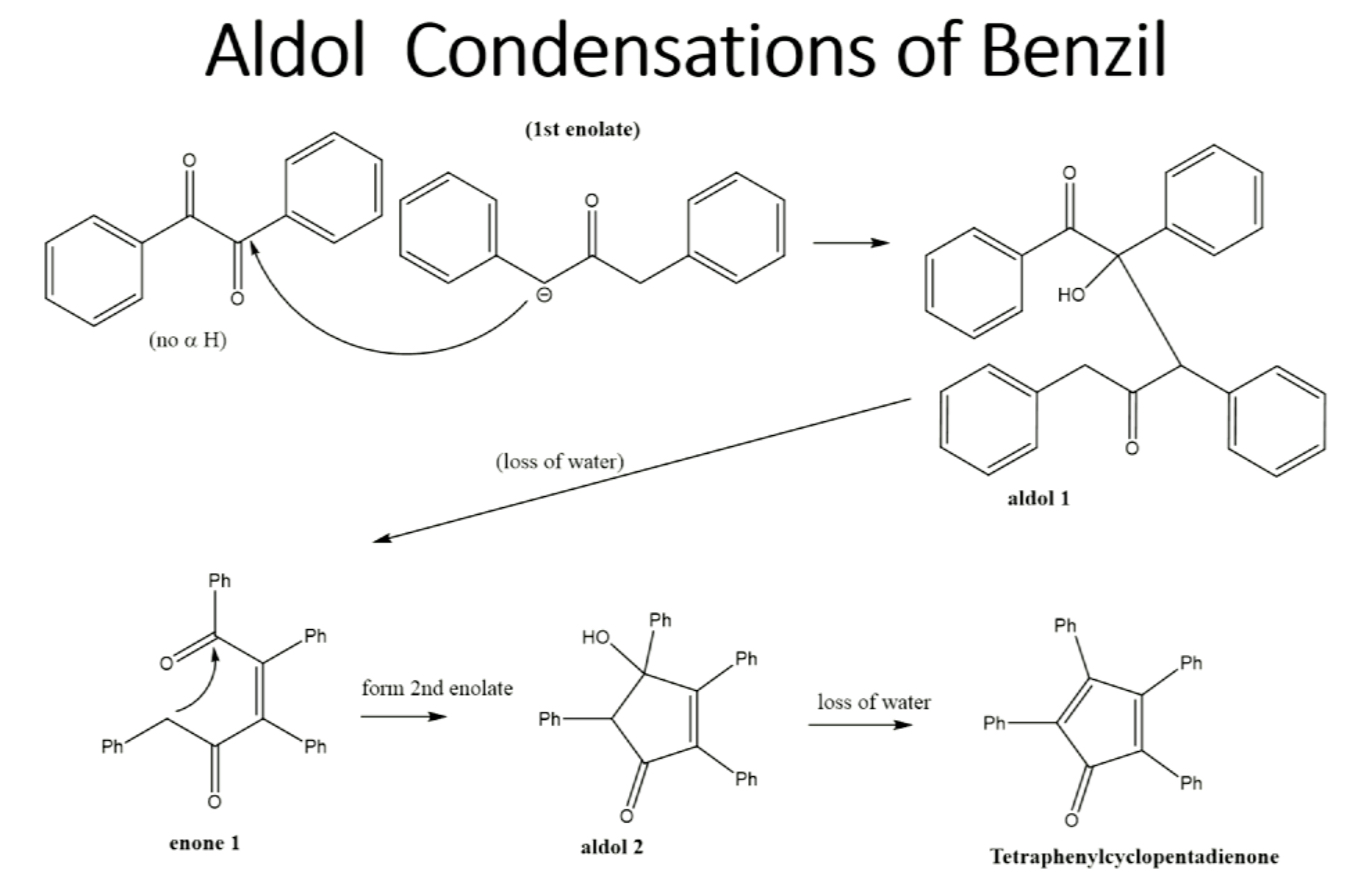
Reaction for step 2 (reactants, reagents, products)
benzil and 1,3-diphenylacetate reacting with KOH to produce tetraphenylcyclopentadienone
Reaction for step 3 (reactants, reagents, products)
anthranilic acid reacting with isopentyl nitrite & 1,2-dimethoxyethane to produce 1,2,3,4-tetraphenylnaphthalene (benzyne, CO2, & N2 produced in rxn)
mechanism for aldol condensation of benzil (step 2)
how should a thermowell be plugged in?
into the variable outlet so you can control the heating
____ will have a higher Rf on the TLC plate because ____
benzil will have a higher Rf on the TLC plate because benzoin is more polar than benzil- the OH group can do H-bonding
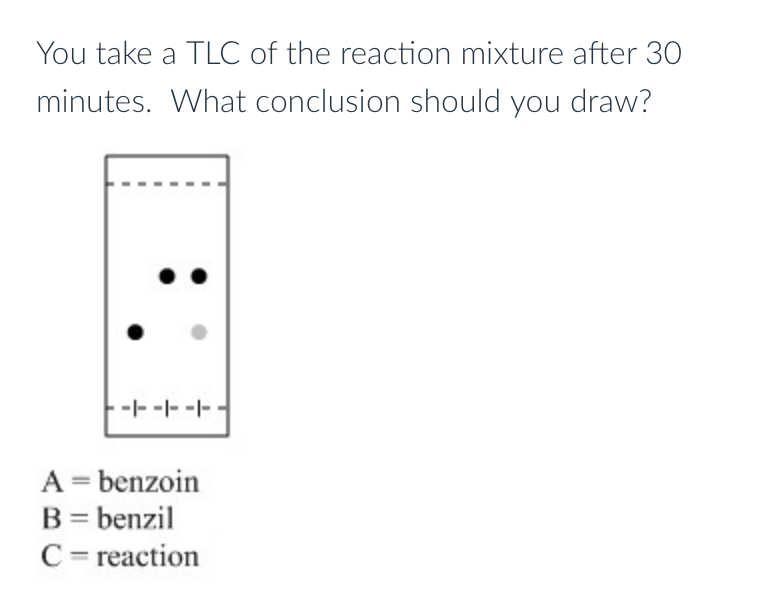
there is both benzoin and benzil present, you should let the reaction continue
what is the stoichiometric (not catalytic) oxidant in this reaction?
ammonium nitrate
if you synthesized 110 mg benzil in the first step, how many mg of diphenylacetone do you need in step 2?
110 mg
if you synthesized 110 mg benzil, how many ml KOH should you use?
0.55 ml
if you synthesized 110 mg benzil, how many ml ethanol should you use?
1
what gases are produced at some point during the synthesis?
carbon monoxide, CO2, N2, nitrogen oxides
is the formation of benzyne from anthranilic acid spontaneous?
yes
what factor is favorable towards making the formation of benzyne spontaneous?
entropy (increases the overall disorder)
what are the three stages in step 3 of the reaction?
stage 1: make benzyne
stage 2: diels-alder reaction
stage 3- eliminate CO
what does stage 1 of step 3 consist of?
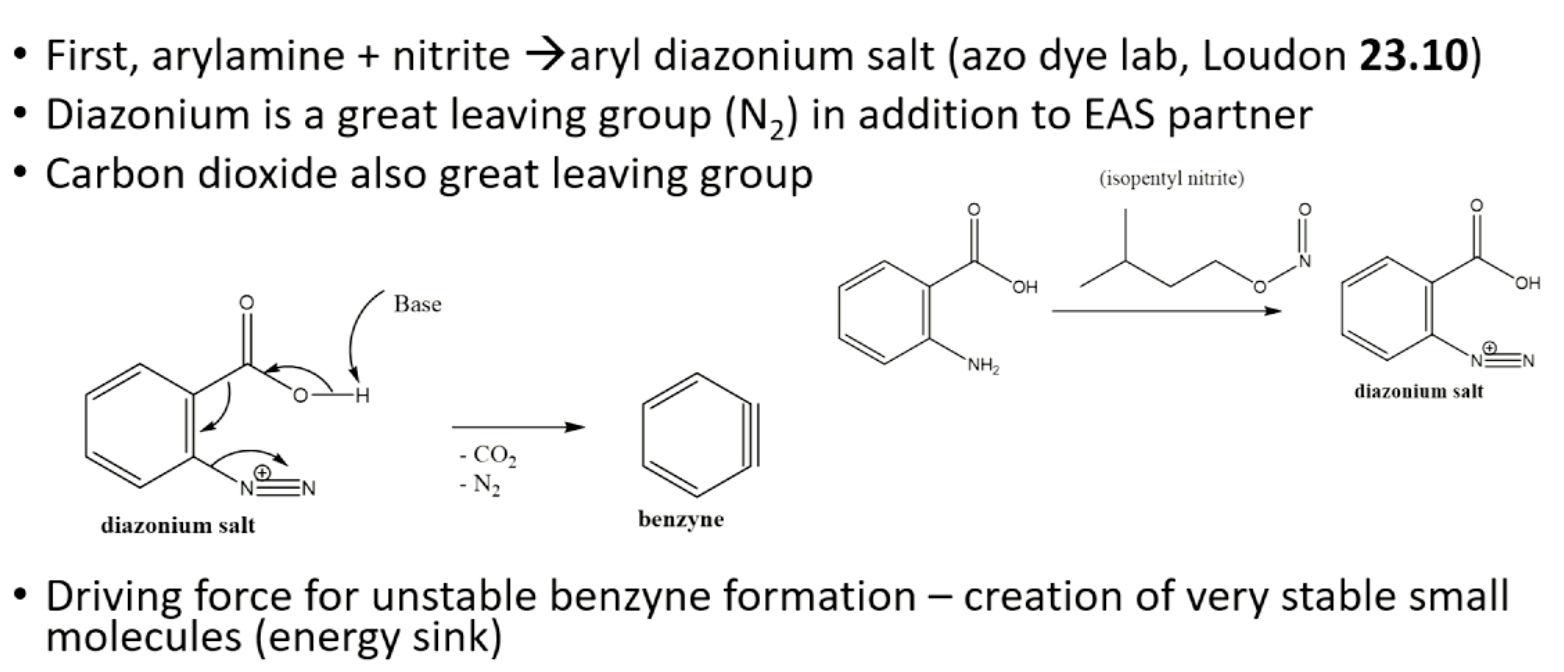
what does stage 2 of step 3 consist of?
what does stage 3 of step 3 consist of?

B (not A because in 241, the diazonium salt was very unstable so the step needed to happen super fast, but in 242, as soon as you make the diazonium, you lose CO2 and N2 producing benzyne, so the step doesn’t need to be as fast and require activated compounds)

B & C
what is Huckel’s rule
determines if a compound is aromatic:
nonaromatic: contains an sp3
anti-aromatic: no sp3, the number of electrons contributing to system is multiple of 4
aromatic: no sp3, the number of electrons contributing to the system is not a multiple of 4