BIO Ch.4 movement of substances across the cell membrane
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
What is diffusion?
Passive movement
when there is a difference in concentration of particles between two regions (concentration gradient)
Net movement of particles down the concentration gradient
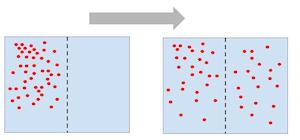
How does the particles move when they are spread evenly throughout the space they occupy?
Reach equilibrium
No net movement
Still move randomly in all direction
how do different particles (non-polar, polar, macromolecules) diffuse across the cell membrane?
Non-polar: directly diffuse through the phospholipid bilayer (simple diffusion)
✅kinetic energy ❌energy from respiration in cells
Example: oxygen, carbon dioxide
Polar: diffuse through the channel proteins or carrier proteins, repelled by the phospholipid bilayer (facilitated diffusion)
✅kinetic energy ❌energy from respiration in cells
Example: glucose, amino acids
What factors affect the rate of diffusion?
Difference in concentration between two regions (how steep the concentration gradient is)
⬆steeper the gradient ⬆ rate
Distance over which diffusion takes place
⬇distance ⬆rate
Surface area of the membrane
⬆surface area ⬆rate
Temperature
⬆temperature ⬆kinetic energy ⬆rate
Size and nature of particles
⬇size ⬆rate
Non-polar ⬆rate
Examples of diffusion.
Gas exchange in the lungs
Absorption of digested food in the intestines
distribution of substances within cytoplasm
What is osmosis?
Net movement of water molecules from a region of higher water potential to a region of lower water potential
across a differentially permeable membrane
water molecules move across the cell membrane through specific channel protein for water
What is water potential?
tendency of water molecules to defuse from one place to another
Water potential: <= 0 (highest: distilled water)
⬆concentrated the solution ⬇water potential(inversely proportional)
How is cells in isotonic solution?
No net movement of water molecules into or out of the cells.
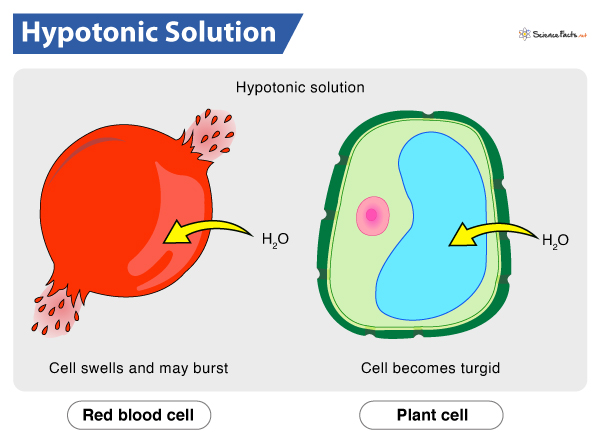
How is cells in hypotonic solution (high water potential)?
water enter the cell by osmosis
Animal cells: swell (膨脹) and eventually burst
Example: haemolysis: bursting of red blood cells leading to the release of haemoglobin
Plant cells: turgid (硬漲) and will not burst
Strong and rigid cell wall prevent cells from bursting
No more water can enter this plant cell when the cell membrane is pressed firmly against the cell wall
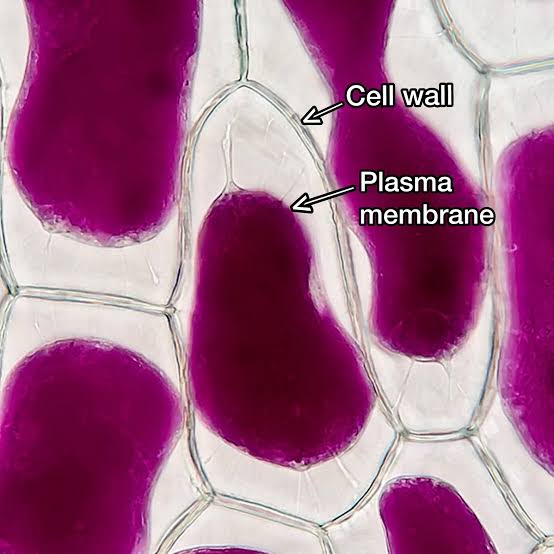
How are cells in hypertonic solution (low water potential)?
Water leaves the cells by osmosis
Animal cells: shrink and become wrinkled
Plant cells: plasmolysed and become flaccid (軟縮)
Cytoplasm and the vacuole shrink due to water loss
The cell membrane detached from the cell wall (plasmolysis 質壁分離)

What is the importance of osmosis?
Animal (human): most of the water in food is absorbed by osmosis in the small intestine
Plant: provide support in young seedings and non-woody plants
gain water: turgid and press against the other cells
Lose water significant: flaccid and wilts (萎蔫)
How does osmosis occur in the onion cells in the hypertonic solution (sucrose solution)?
The onion cells are plasmolysed and flaccid
This shows that the water potential of the sucrose solution is lower than that of the onion cells
When water leaves the onion cells, they become flaccid and plasmolysed
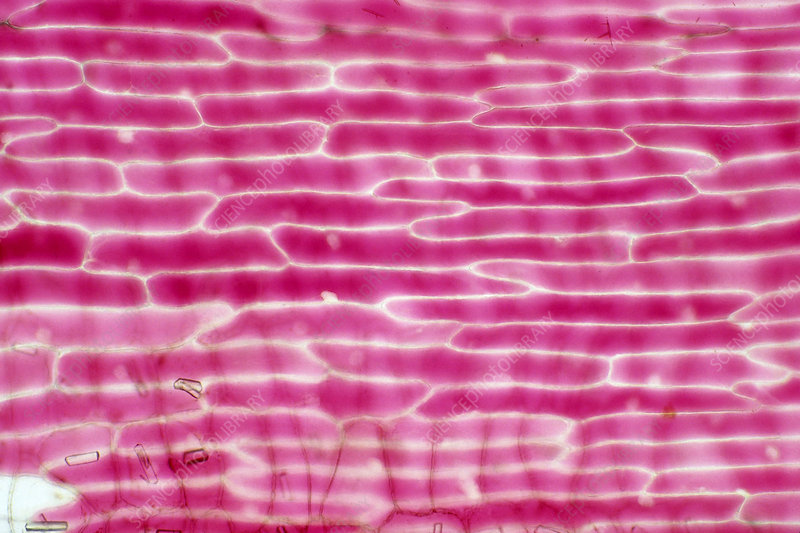
How does osmosis occur in the onion cells in the hypotonic solution (distilled water)?
The onion cells become turgid
This shows that the water potential of the distilled water is higher than that of the onion cells
When water enters the onion cells, the onion cells become turgid
How does osmosis occur in the red blood cells in the isotonic solution?
The red blood cells appear normal
This show said there is no difference in the water potential between the cells and their surroundings
There is no net movement of water into or out of the cells
Why do we have to put a cover slip over the epidermis?
Prevent evaporation of water from the sucrose solution
change the water potential
affect the experimental result
Flatten the specimen
Prevent the object from touching the specimen
what are the applications of osmosis?
Soaking food in a solution with high salt or sugar concentration (hypertonic solution)
Remove water from the food and microbes by osmosis
Use slightly hypertonic solution to store red blood cells
Prevent cells from drawing in water and bursting
Isotonic saline solution to dissolve drugs for intravenous injection (靜脈注射)
What is active transport?
Substances are absorbed against the concentration gradient
usually occur when plants absorb mineral ions from the surrounding
How does active transport occur?
✅energy from respiration ❌kinetic energy
⬆rate of respiration ⬆rate of active transport
In living cells only
Transported through specific carrier proteins
Example of active transport.
Active minerals from the soil into the roots of plants
We absorption of glucose and amino acids in kidney tributes
Absorption of small water soluble molecules (e.g. monosaccharides, minerals) in small intestine
What is phagocytosis?
Large particles cannot enter cells by the diffusion or active transport (so use phagocytosis)
How does phagocytosis occur?
The cell membrane folds in to form a pit or it extends out to form pseudopodia
The particles is enclosed by vacuole and is taken into the cell
A lysosome containing enzymes move towards the vacuole
The digested products diffuse into the cytoplasm
The lysosome fuses with the vacuole. The particle is digested by the enzymes
Example of phagocytosis.
White blood cells can engulf invading microorganisms by phagocytosis
Some unicellular organisms use phagocytosis for feeding
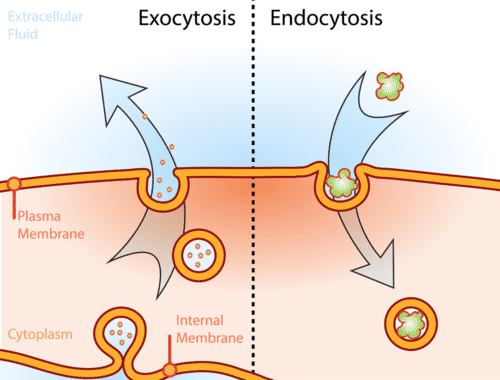
What is endocytosis (胞吞) and exocytosis (胞吐)?
Endocytosis: intake of minerals into the cells by the infolding of the cell membrane to form a vacuole (e.g. phagocytosis)
Exocytosis: removing wastes or release secretory products by enclosing them in vesicles which then move to the cell surface for release