Atomic Theory, Molecular Mass, and Conversions in Chemistry
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
What is the atomic mass of an element?
The weighted average mass of atoms of the element, measured in atomic mass units (amu).
What is molecular mass?
The sum of the atomic masses for each element in a molecule or compound, also measured in amu.
What is molar mass?
The mass of one mole of a substance, measured in grams per mole (g/mol).
How is the molar mass of monatomic elements determined?
The molar mass is the same as the atomic mass but expressed in grams per mole.
What is the molar mass of Neon?
20.18 g/mol, which is the same as its atomic mass of 20.18 amu.
What is the relationship between molecular mass and molar mass for compounds?
The molar mass is the same as the molecular mass but expressed in grams per mole.
How do you convert between mass and moles of a substance?
Use the molar mass to determine the mass of a given number of moles or vice versa.
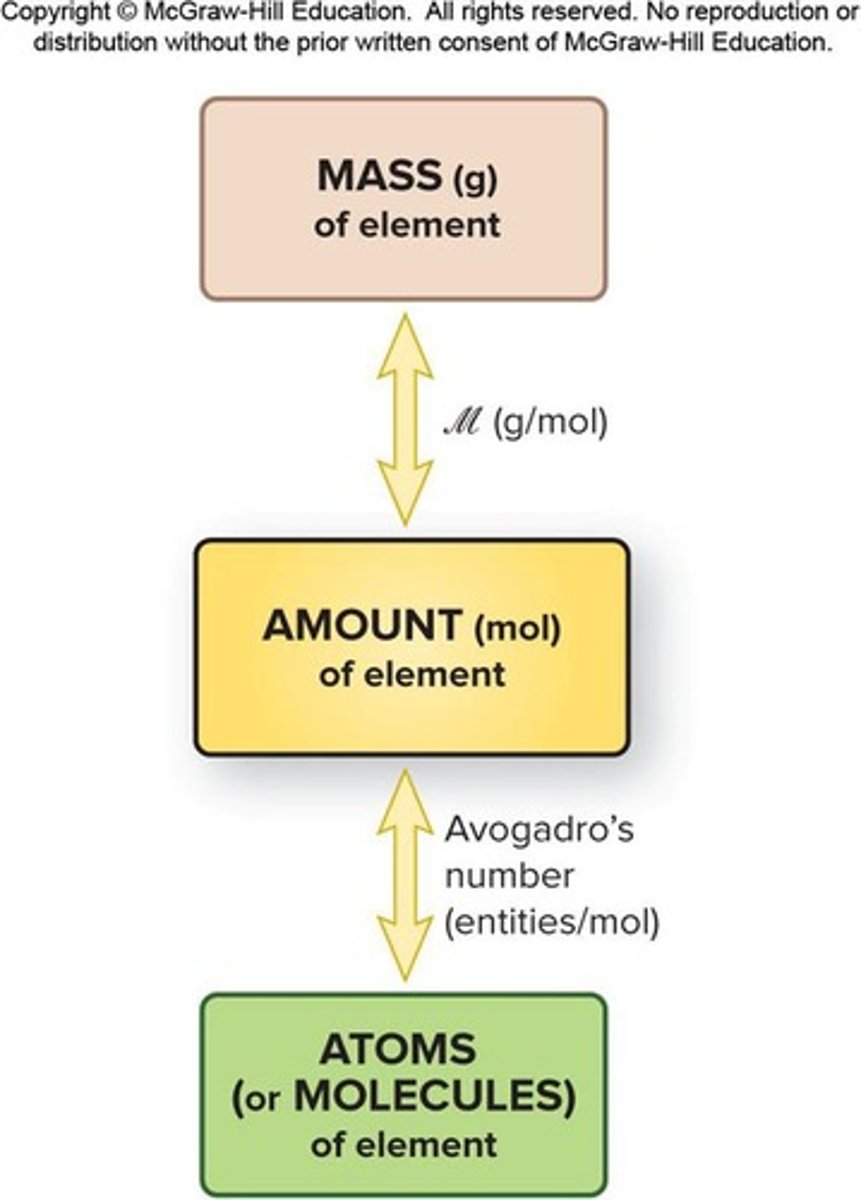
What is the formula for calculating molecular mass?
Molecular mass = sum of atomic masses of all atoms in the molecule.
What does the term 'formula mass' refer to?
It is another name for molecular mass, calculated from the sum of atomic masses.