Science Chemical Bonds
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Ionization Energy
The energy required to remove an electron from a neutral atom
Electronegativity
The ability of an atom to attract electrons
Ion
An atom that has gained or lost electrons
Octet Rule
Atoms tending to gain, lose, or share electrons in order to have a full valence shell of 8 electrons
Chemical Bond
The attractive force between atoms that binds them together as a unit, having one of these will decrease the potential energy of these atoms and increase their stability
Ionic Bond
Bond formed between a metal and a non metal that become ions due to the transfer of electrons
Characteristics of atoms in an ionic bond
Conduct electricity, dissolve in water, form crystals, and have increased melting points
Covalent Bond
Bond formed by the sharing of electron pairs
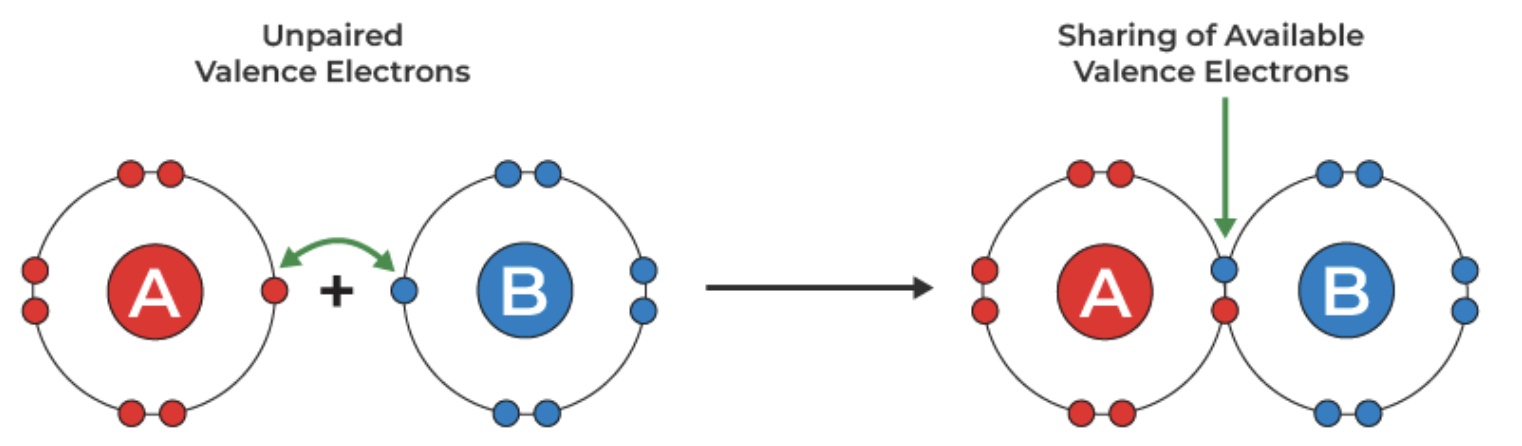
Characteristics of atoms in a covalent bond
Will NOT conduct electricity, dissolve in water, form crystals, and will have decreased melting points. These will also be odorous.
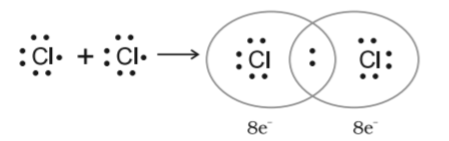
Non-polar Covalent Bond
When two atoms share electrons equally
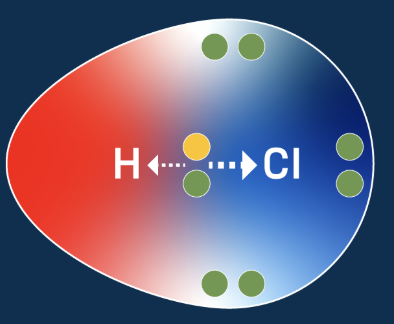
Polar Covalent Bonds
When electrons are shared, but not equally
Up and to the Right
The ionization energy and electronegativity of an atom increases going _____, meaning non-metals have more of these qualities because they are the “takers”
Bigger atoms
It is easier to take electrons from _____ because their valence electrons are farther from the nucleus
Ionic
The greater the difference in electronegativity between atoms, the more ____ the bond becomes
Subscript
a small number written to the right and slightly below a chemical symbol to indicate the number of atoms of that element present in a molecule or compound
Oxidation State
the hypothetical charge an atom would have if all its bonds to different atoms were ioni
Cation
Atom with a positive oxidation state
Anion
Atom with a negative oxidation state
Polyatomic Atoms
A group of covalently bonded atoms that act as a single ion
Dipole
A region of positive and negative charge in a molecule or formula unit
Chemical Formula
A shorthand representation of a substance using the the symbols for the atoms as well as subscript to indicate the number of each ato