Alkenes and Arenes
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
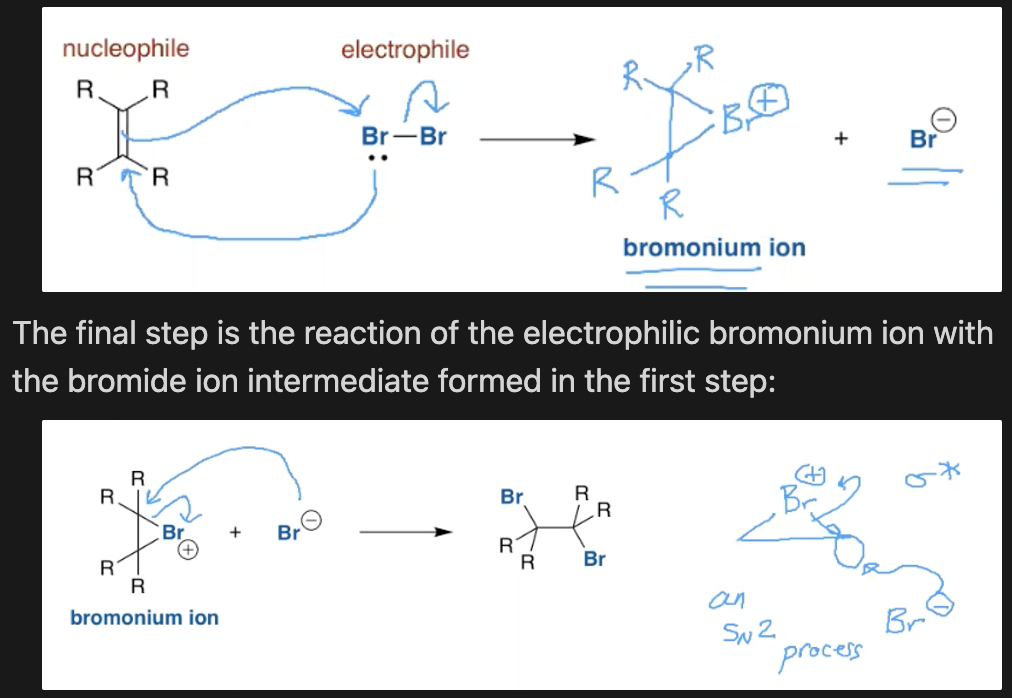
What is the mechanism for the bromination of alkenes?
What are the most common oxidising agents for an alkene?
Peroxy acids (peracids)
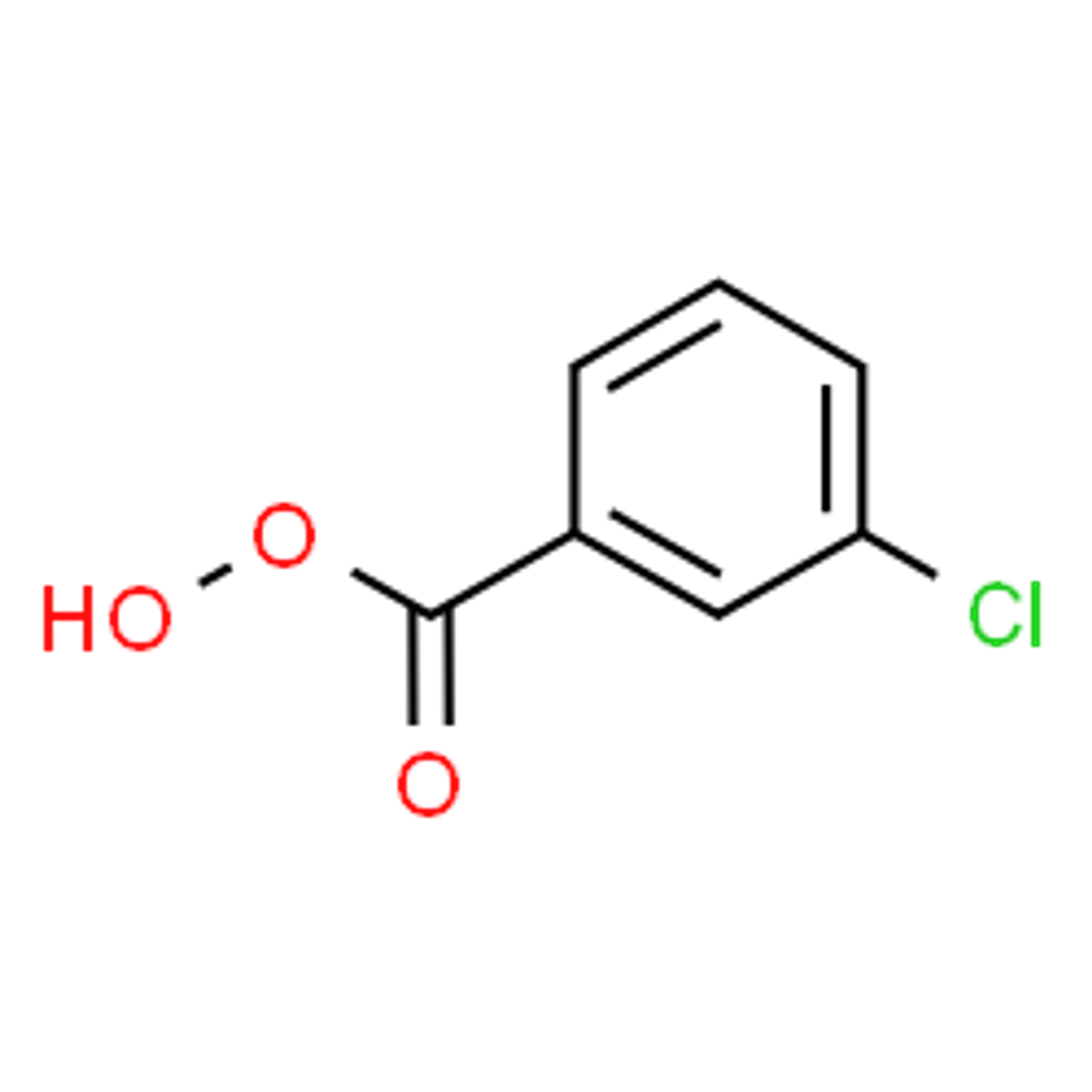
What is the most popular peracid and what is its structure?
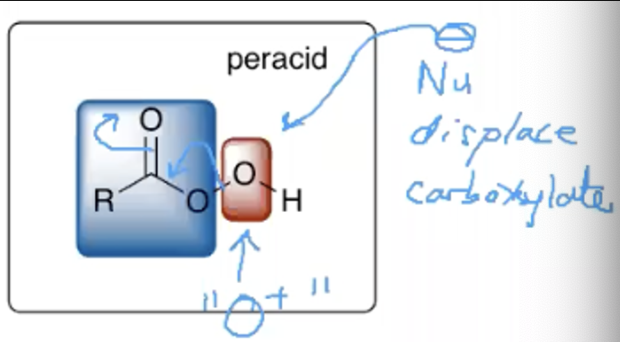
What’s the mechanism for the synthesis of an epoxide?
Alkene
Peracid
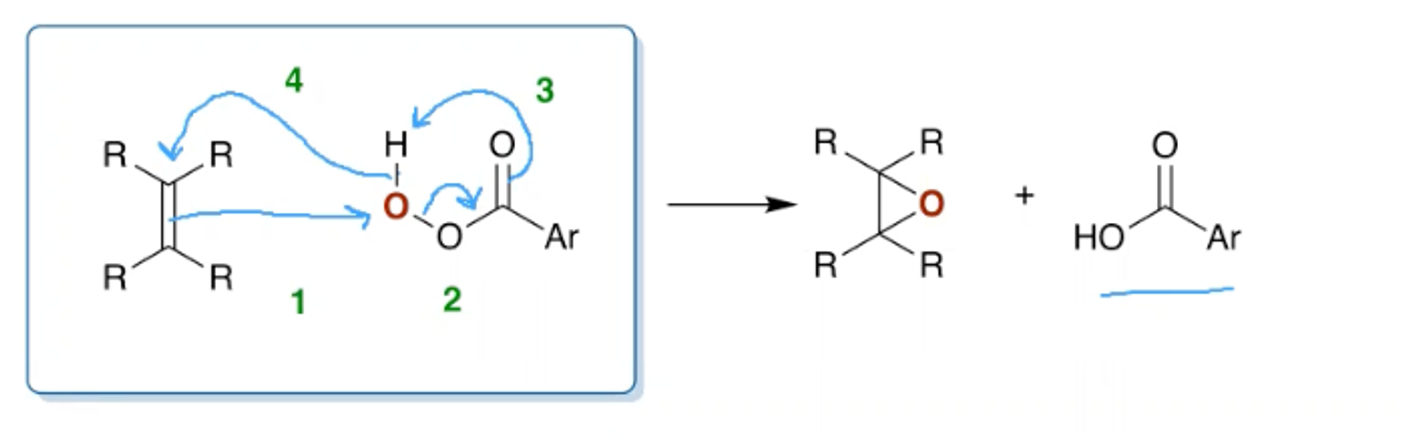
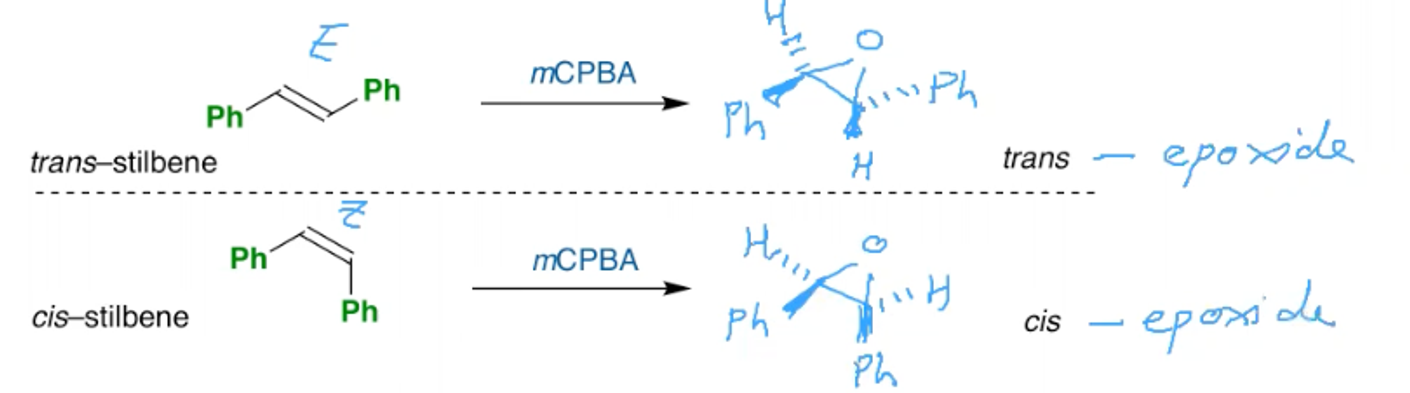
What is the stereoselectivity of the synthesis of epoxides?
A cis alkene will make a cis epoxide
A trans alkene will make a trans epoxide
What effect does having many substituents on the alkene have on epoxidation?
It makes it faster
Alkyl groups are electron donating, stabilising them via hyperconjugation. The more alkyl substituents on the alkene the more electron rich it is and the better the nucleophile it is.
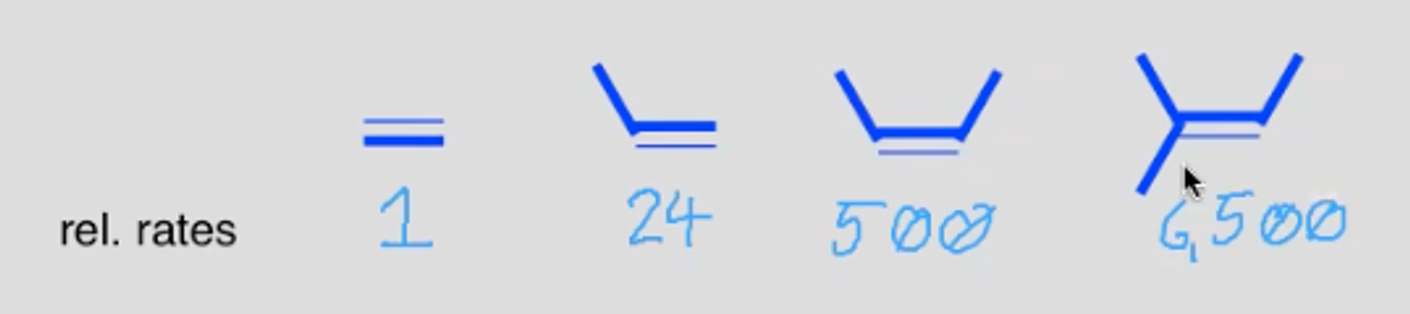
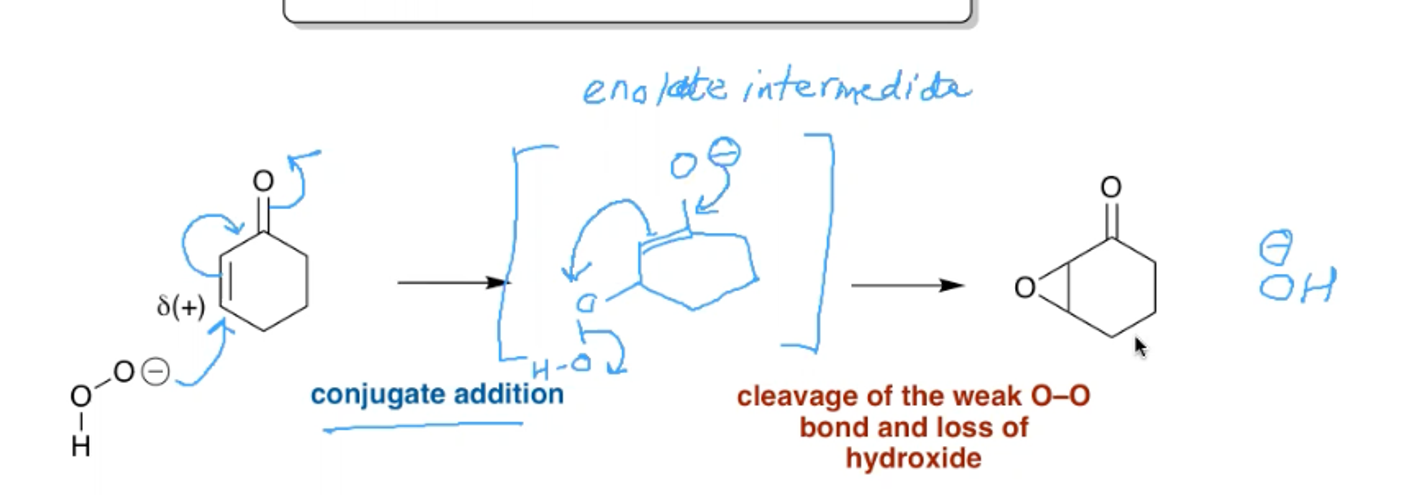
What is the nucleophilic epoxidation reaction? When is it used and what are the reagents?
This mechanism is needed for electron poor alkenes.
You would use hydrogen peroxide and a base as the nucleophile.
In the mechanism outlined above the nucleophile could also attack the carbonyl carbon.
What are the reagents and outcome of the dihydroxylation of an aryl alkene?
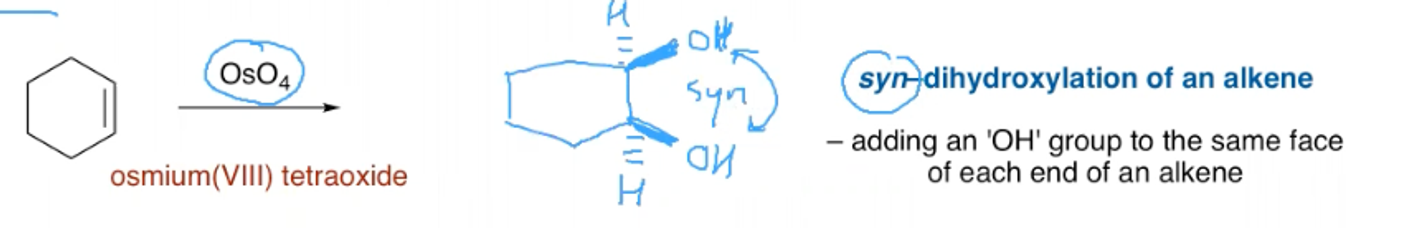
What are the reagents and outcome of the ozonolysis of an aryl alkene?
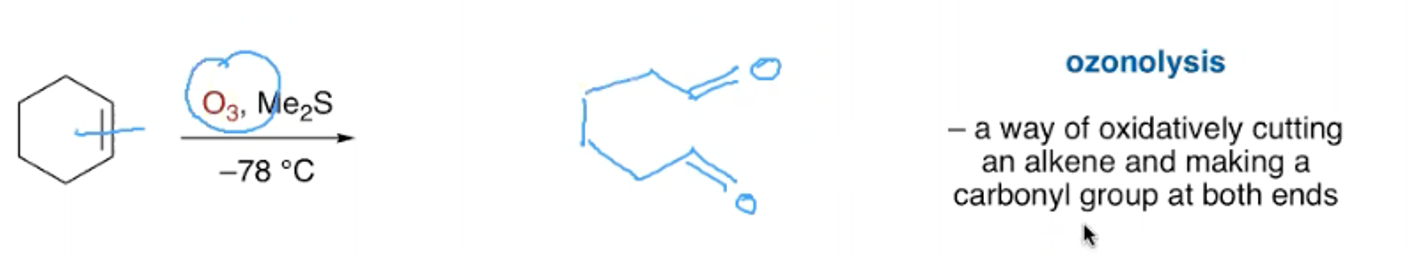
What are the reagents and outcome of the ozonolysis of a non cyclic alkene?
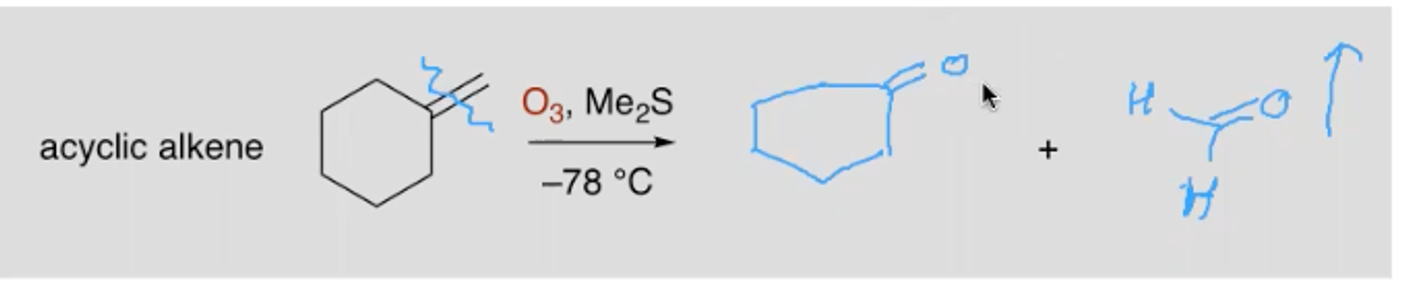
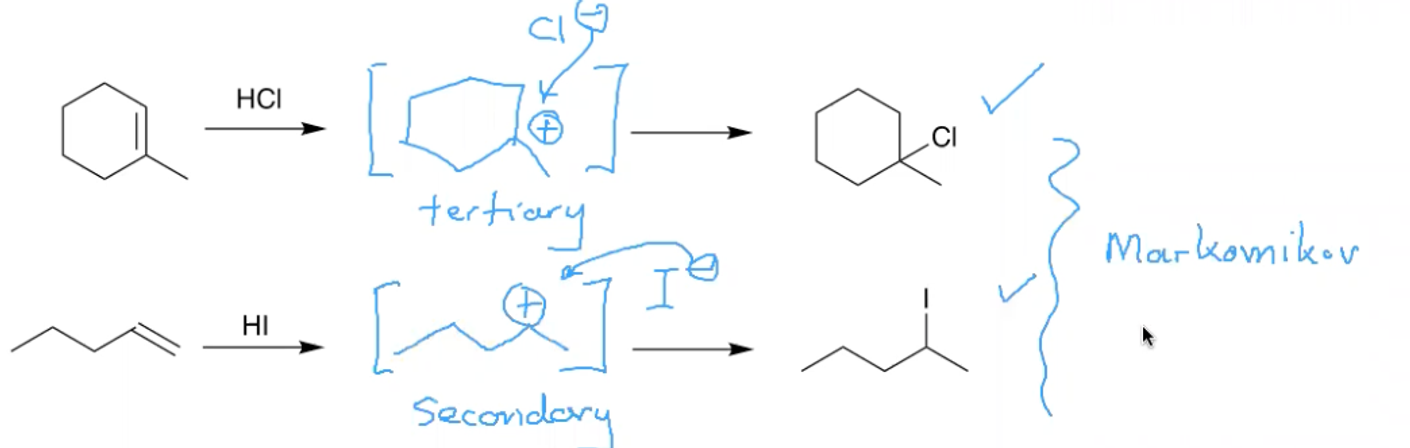
What is Markovnikov’s rule?
In additions of HX to an alkene, the hydrogen attached to the carbon of the double bond that had more hydrogens to start with.
Basically the most stable intermediate possible is formed.
What can be said about the opening of bromonium ions in a nucleophilic solvent?
Solvent molecules compete with the bromide counter ion to open the bromonium ion. This happens in water and alcoholic solvents as even though they are shoddy nucleophiles their conc is so high they outcompete.
They attack the most subbed end of the bromonium ion, unlike the Br counter ion which attacks whatever. This is known as a loose SN2 reaction.
If the solvent is water, the products are bromohydrins and can be converted into epoxides by treatment with base:
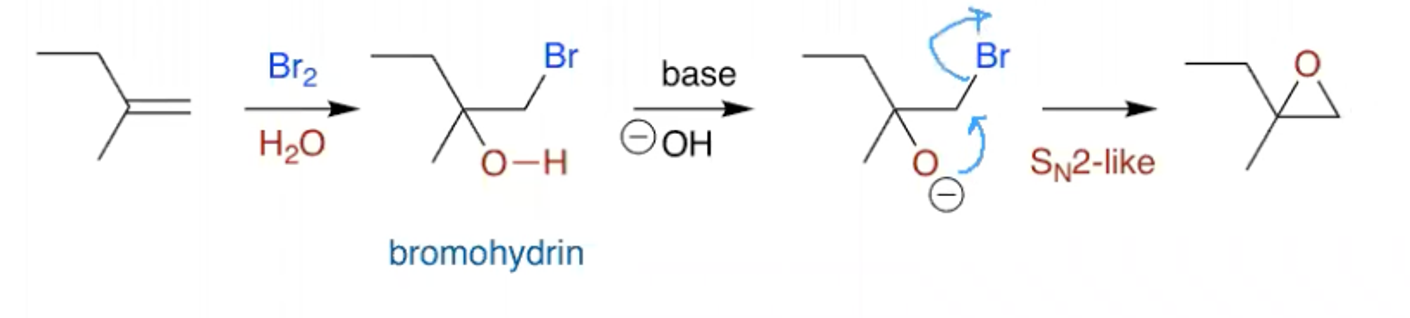
How can you open an epoxide at the most subbed end?
Using HCl and MeOH in a loose SN2 reaction
The protonation produces a positively charged intermediate that acts like a bromonium ion.
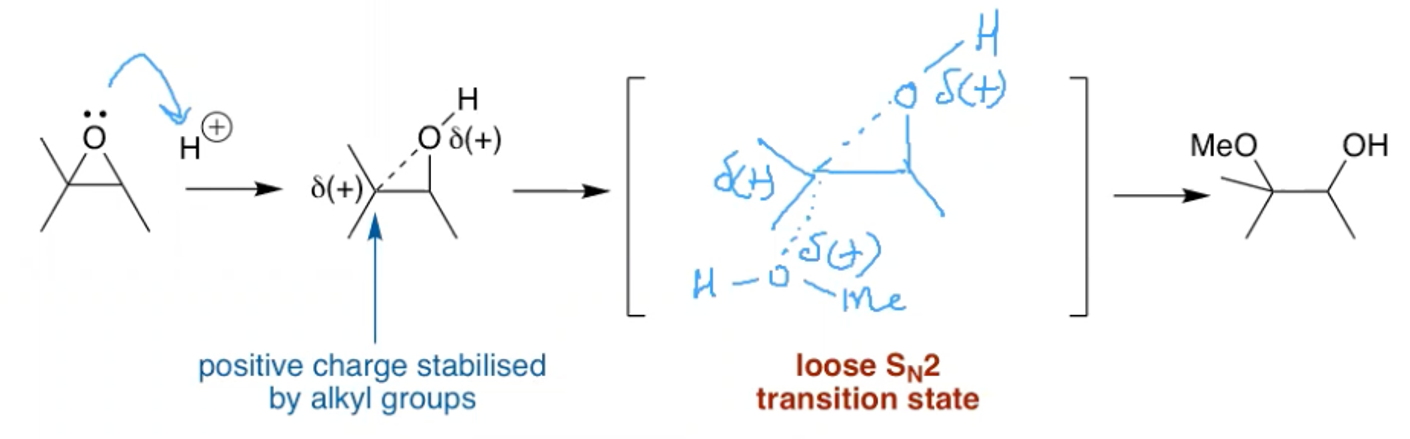
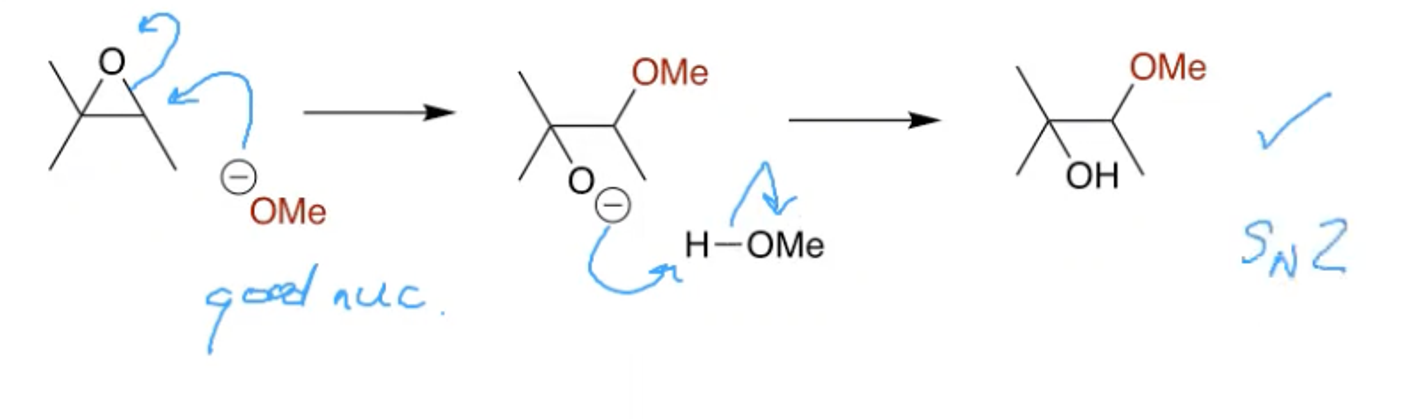
How can you open an epoxide at the less subbed end?
Using MeONa
This is an SN2 reaction and steric hinderance is the controlling factor, this it attacks at the less substituted end of the epoxide.
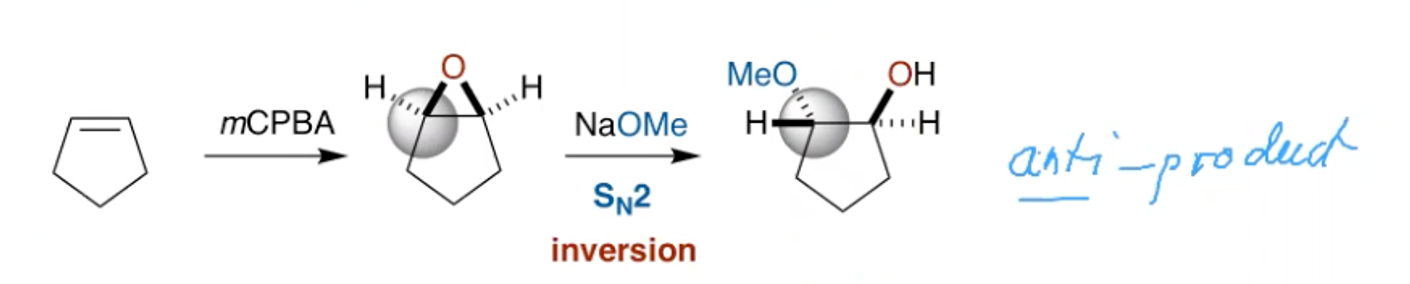
What can be said about the stereochemistry of epoxides and bromonium ions?
The opening of epoxides and bromonium ions proceeds via SN2, resulting in inversion.
What reagent is typically used when doing a bromination?
N-Bromosuccinimide (NBS).
It is a more user friendly source of Br2.
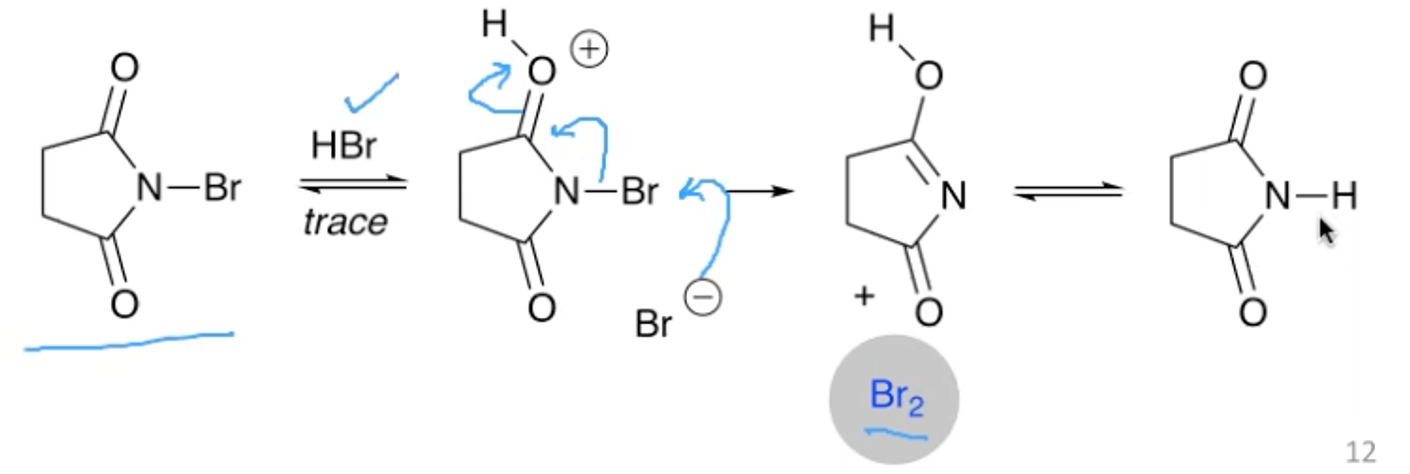
What is the stereochemistry of the products of the bromination of cis and trans alkenes?
Trans alkenes give anti products
Cis alkenes gives syn products
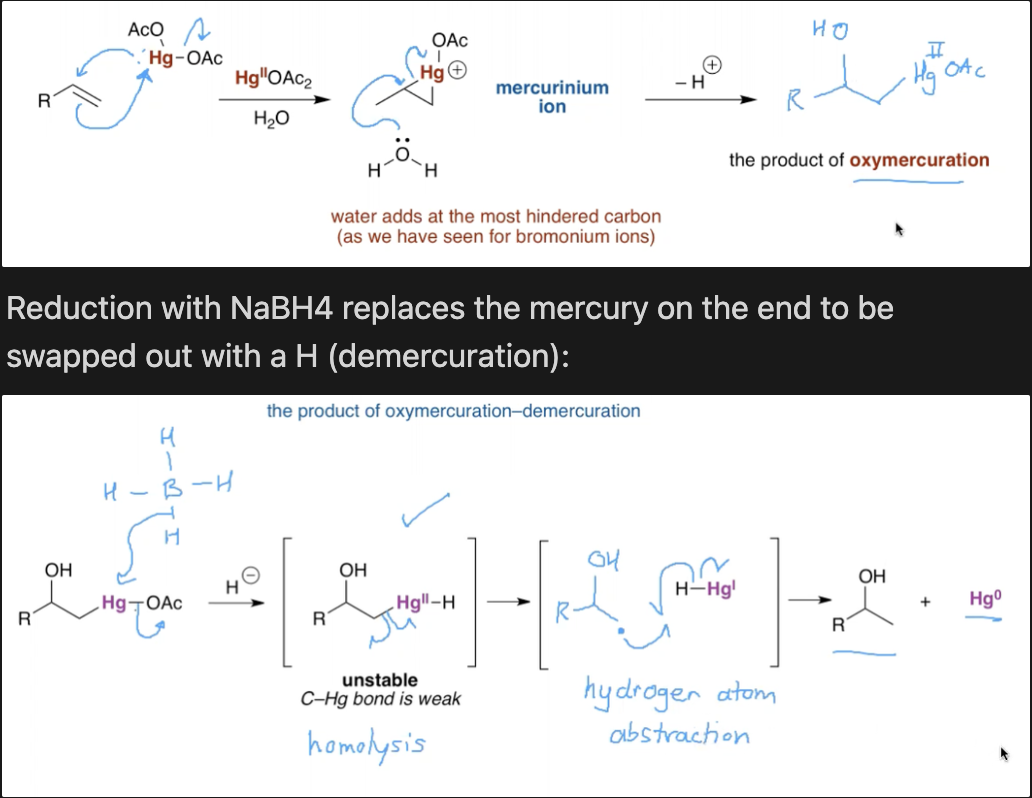
What is the most reliable way to hydrate an alkene? What is the mechanism?
Using mercury
Hg(II)OAc2 + H2O
BH4
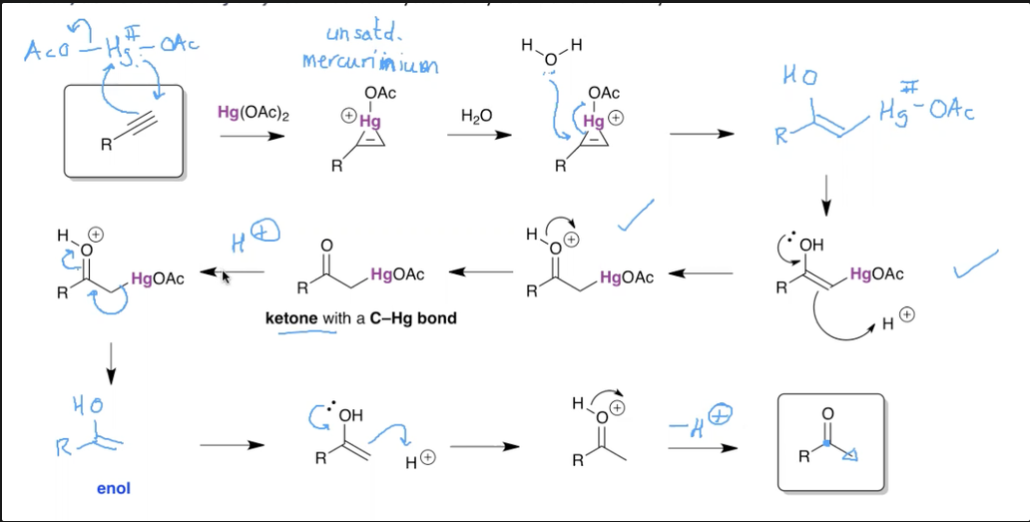
What is the mechanism for the hydration of alkynes using mercury?
Alkyne mechanism does NOT need the BH4.
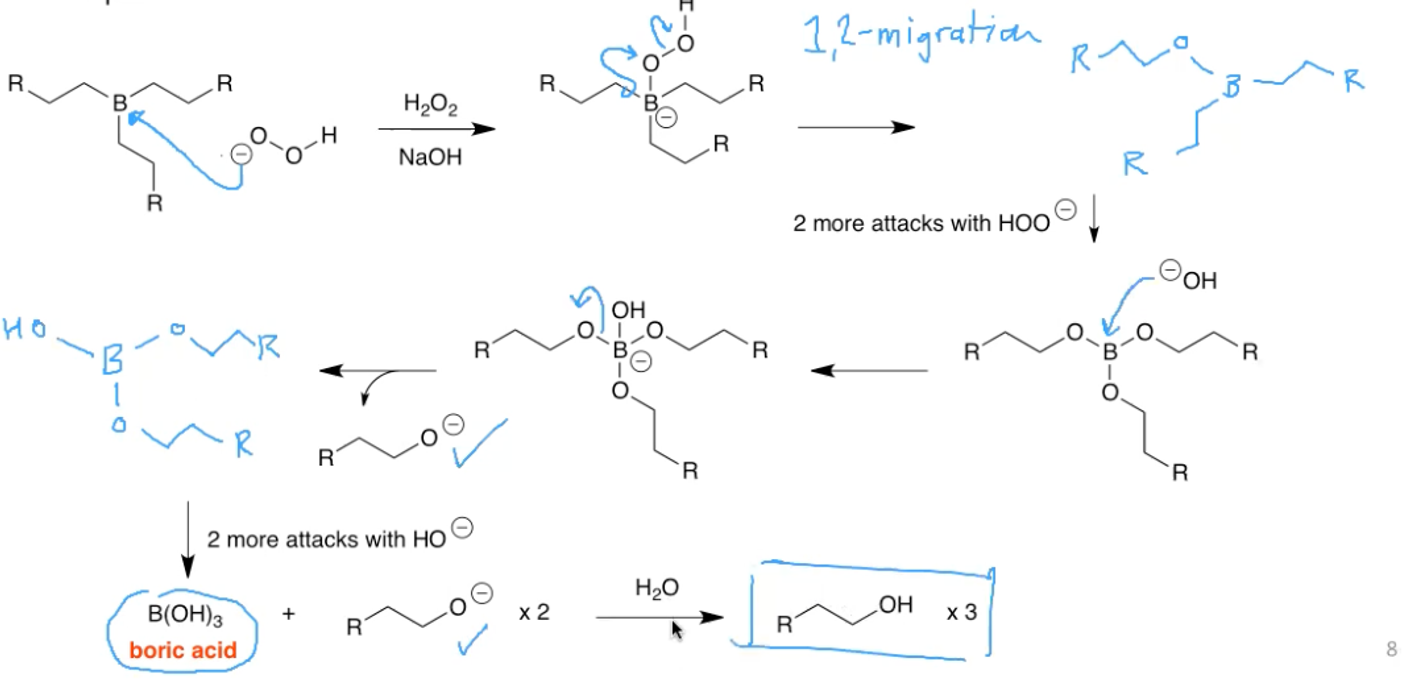
What is the mechanism for the anti Markovnikov addition of water to an alkene?
BH3
H2O2, NaOH
What are the reagents used for the hydrogenation of an alkene?
H2, Pd/C
What are the reagents used for the hydrogenation of alkynes to Z alkenes?
H2, Lindlar’s catalyst
This is Pd supported on CaCO3, deliberately poisoned with Pb(OAc)2
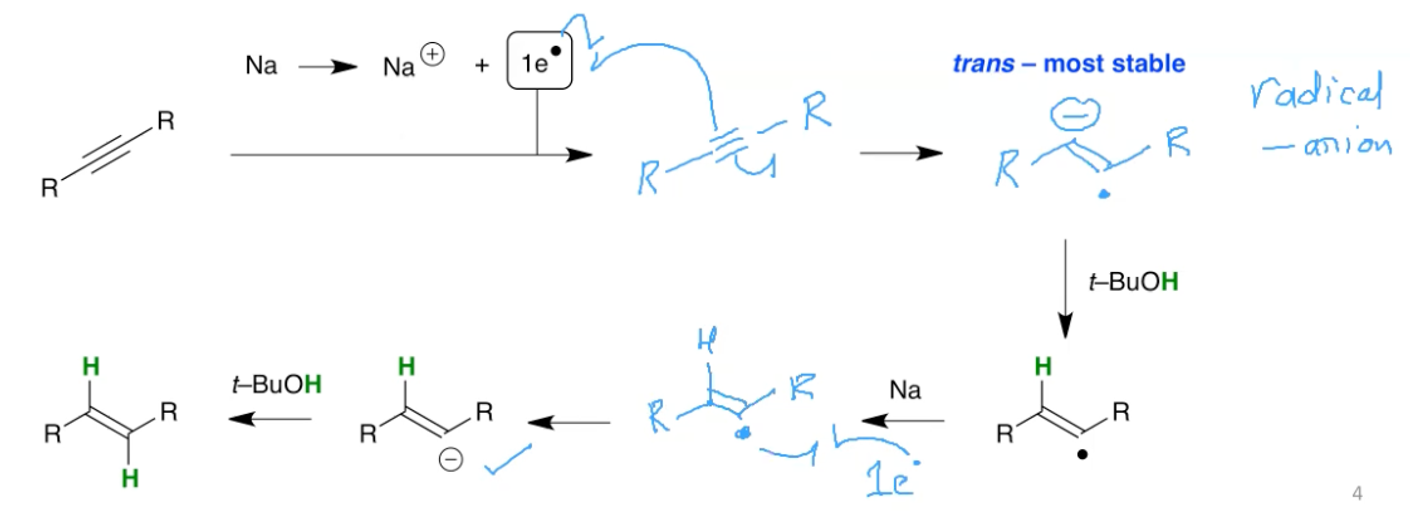
What are the reagents for the hydrogenation of an alkyne to an E alkene? What does the mechanism look like?
Na, NH3, t-BuOH
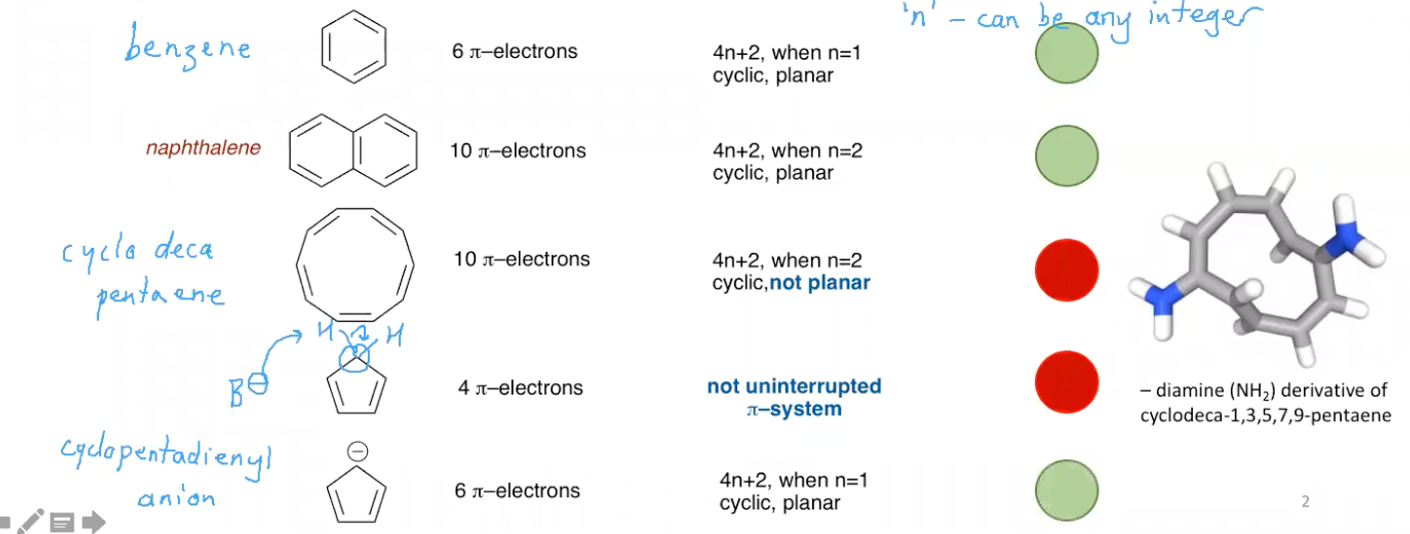
What is Hückle’s rule?
It defines an aromatic compound as a ‘planar, cyclic compound containing an uninterrupted conjugated system with 4n + 2 pi electrons’ Where n is any integer.
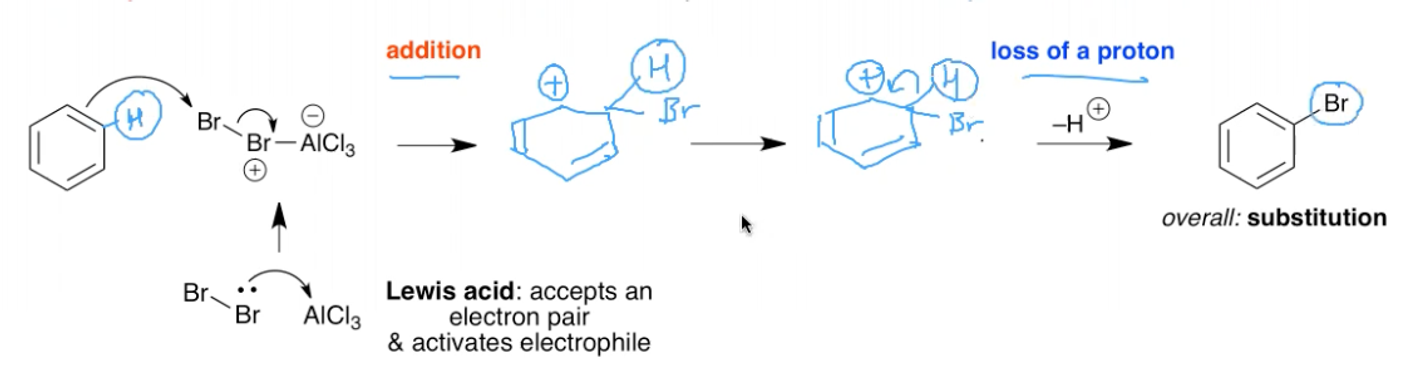
What is the mechanism for the bromination of benzene?
Br, AlCl3
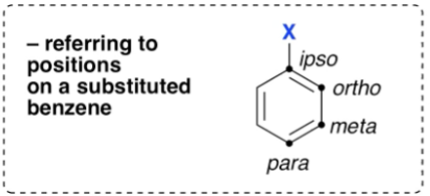
Where are the ipso, ortho, meta and para positions?
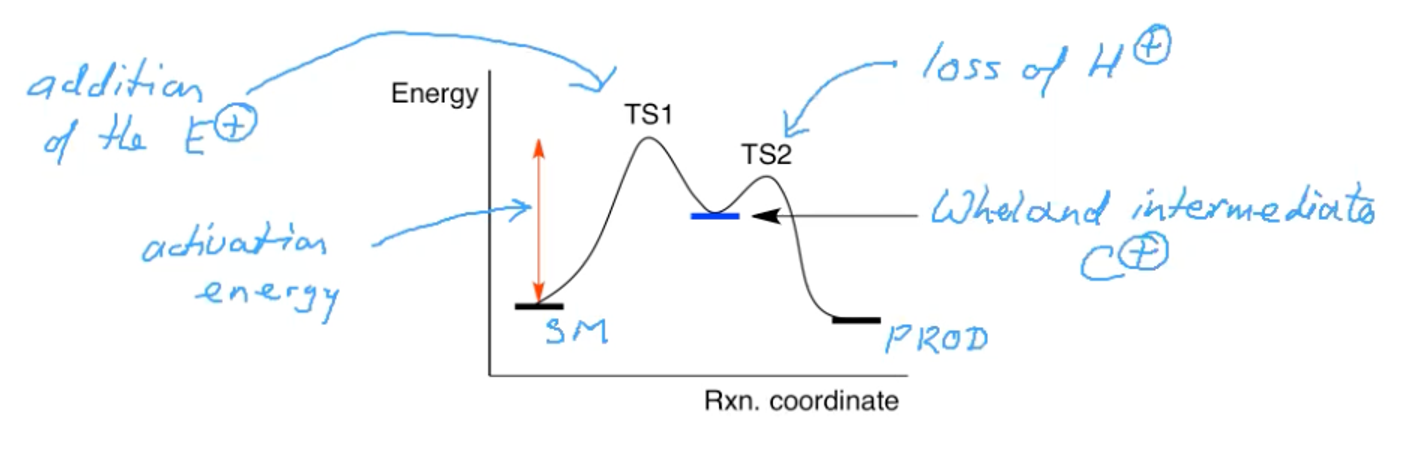
What does the transition state diagram of the addition of bromine to benzene?
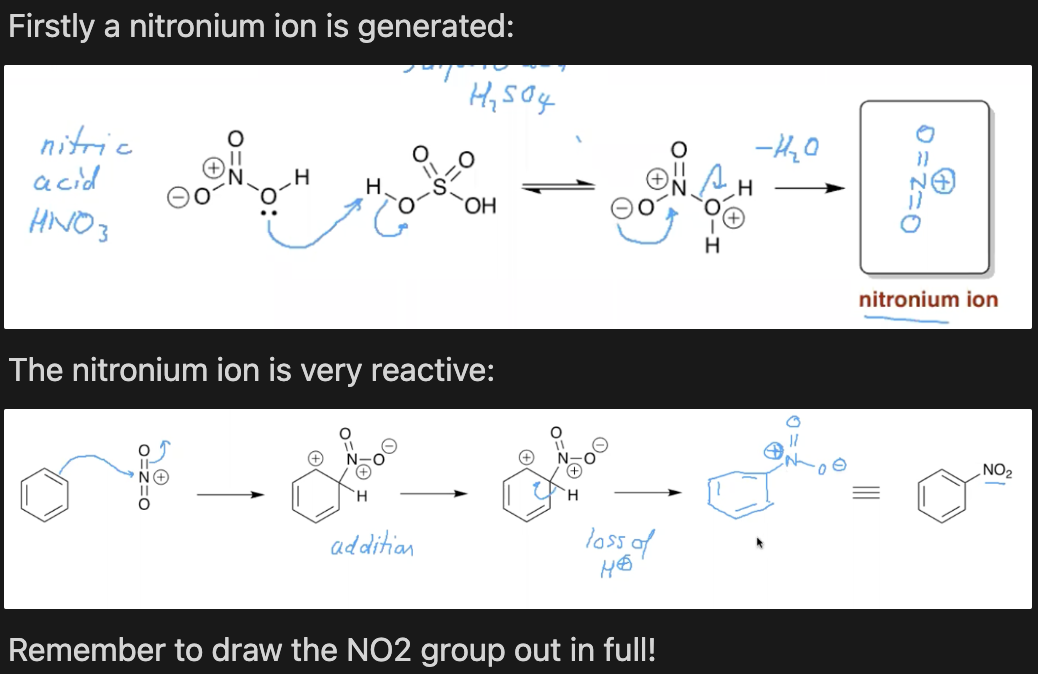
What is the mechanism for the nitration of benzene?
HNO3, H2HO4
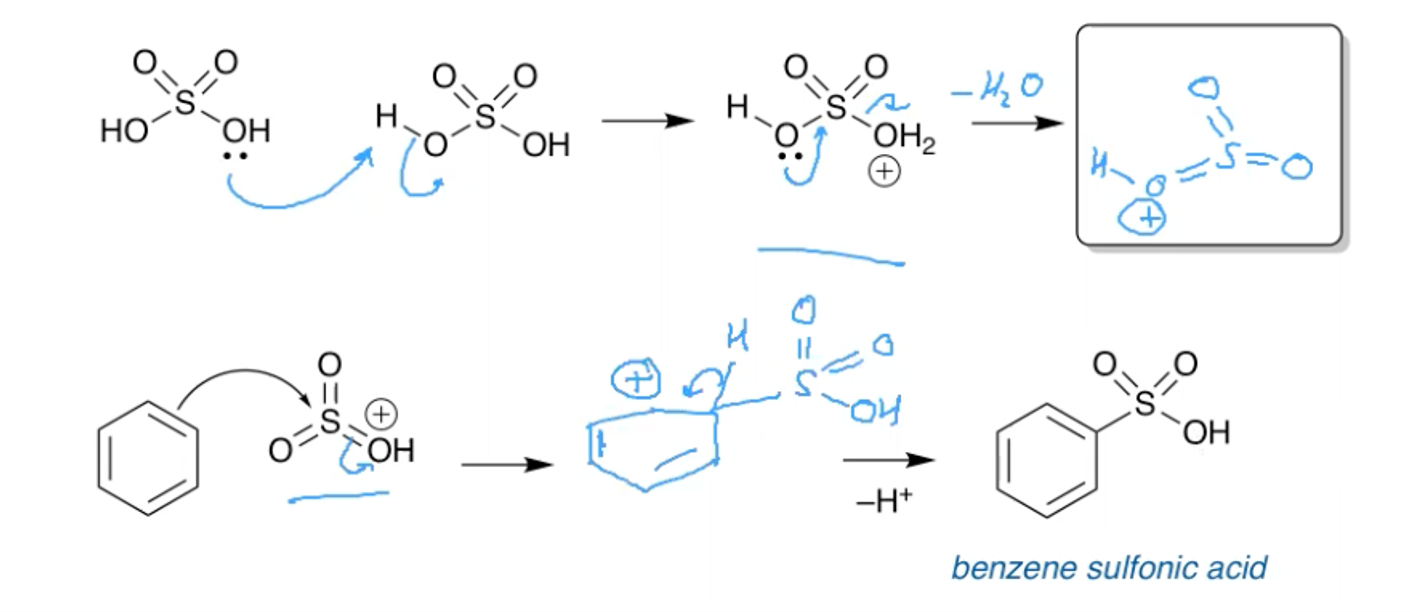
What is the mechanism for the sulfonation of benzene?
H2SO4
Reacts SLOW
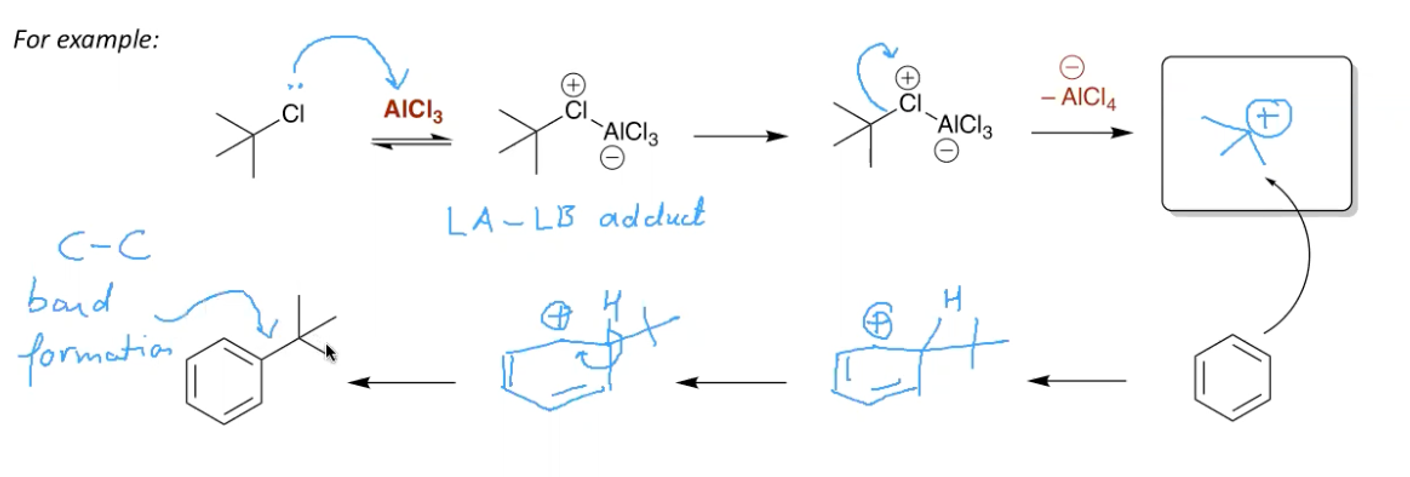
What is the mechanism for Friedel Crafts alkylation of benzene?
non primary alkane with a Cl on it
AlCl3
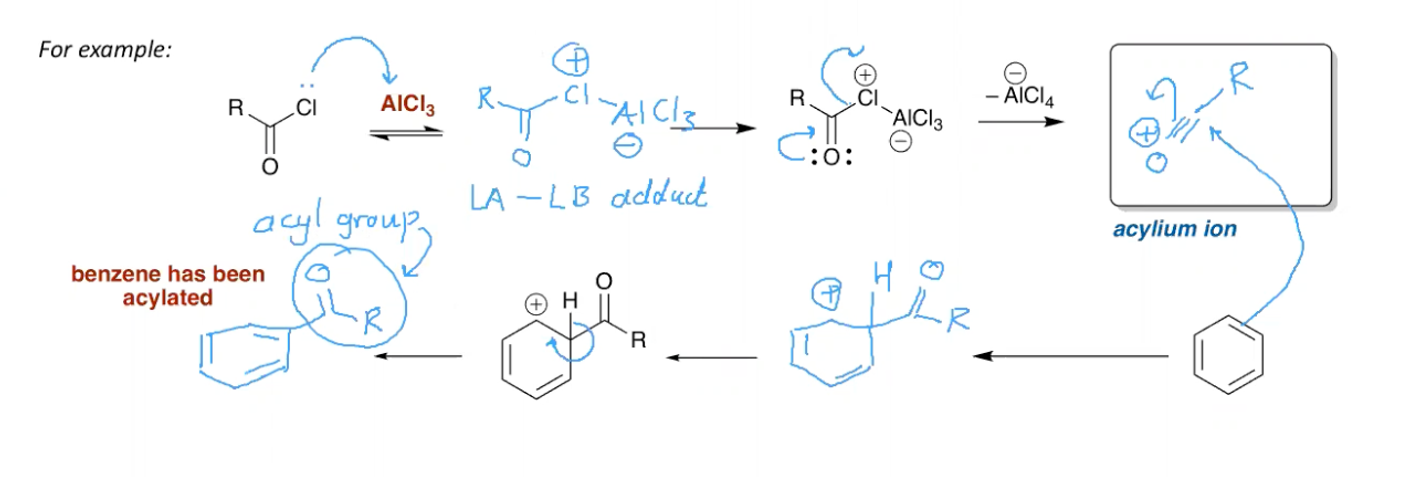
What is the mechanism for Friedel Crafts Acylation?
Acyl Chloride, AlCl3
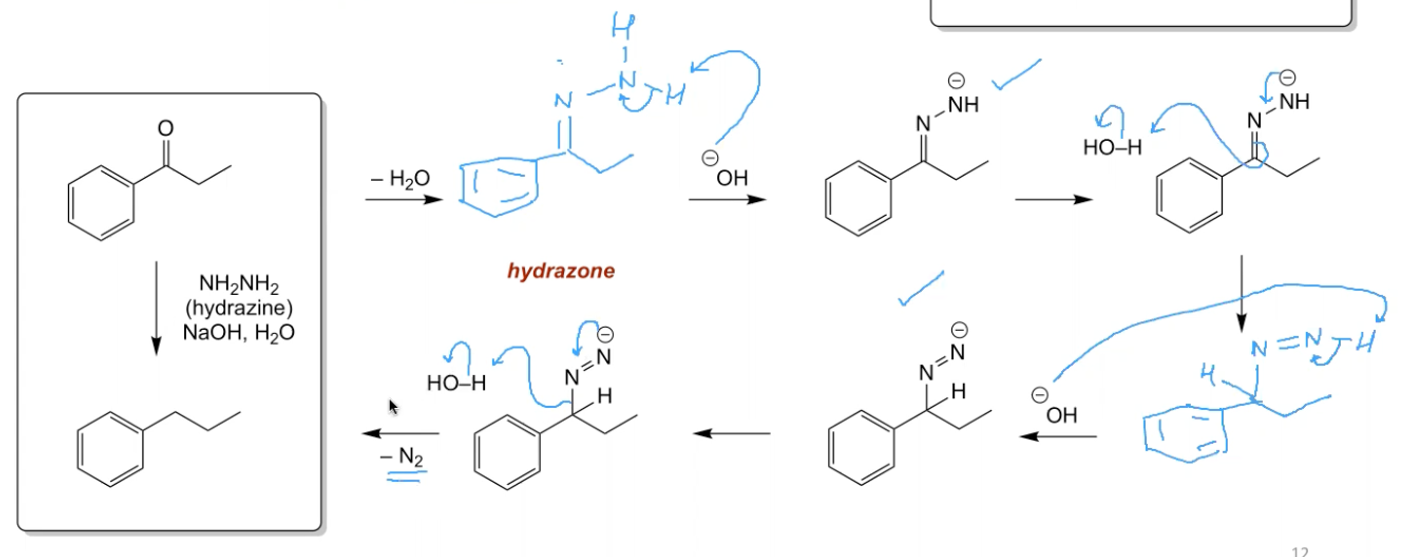
What is the mechanism for Wolff Kishner reduction?
NH2NH2 (Hydrazine)
NaOH, H2O
What is the criteria for a substituent to be an ‘activating’ Ortho-Para director?
It must have a lone pair, for example OH or NH2.
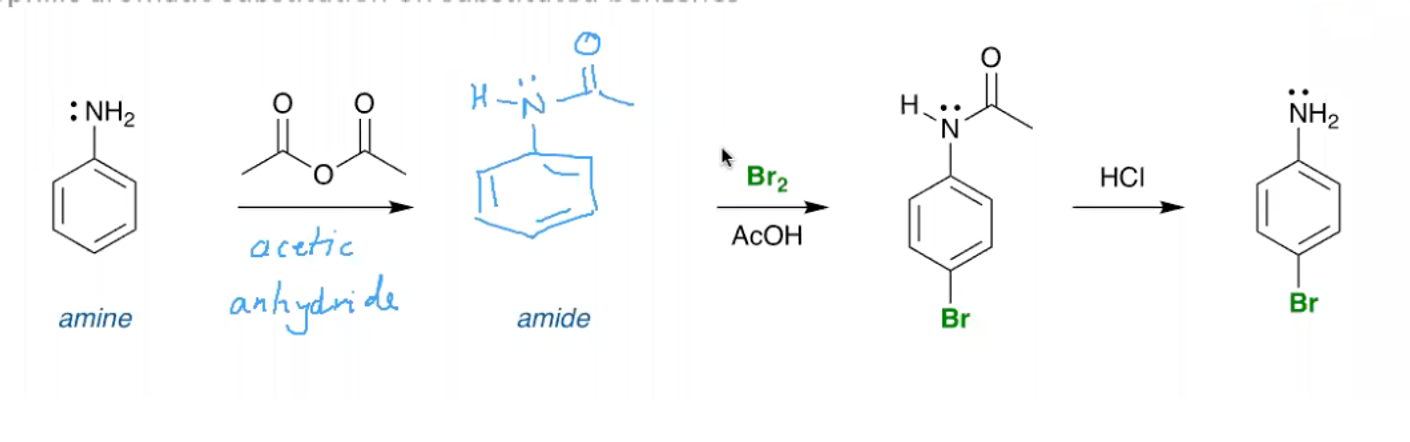
How can we temporarily stunt the reactivity of an aromatic ring with NH2 attached to it?
Using acetic anhydride you limit the availability of the LP on the nitrogen.
You can then use HCl to return the molecule to how it was before.
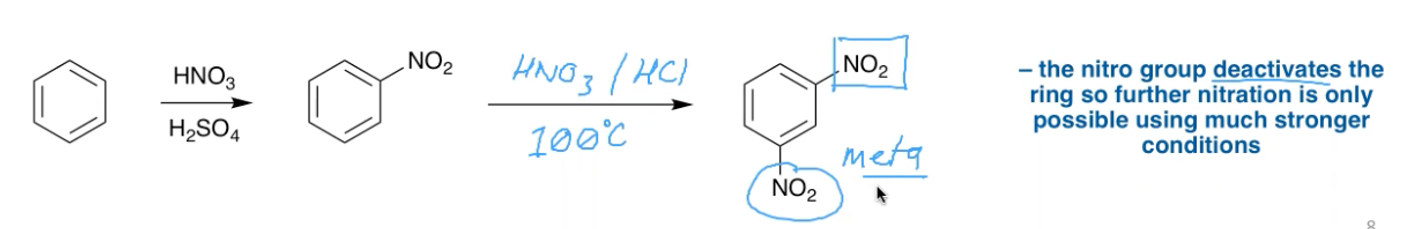
What is the criteria for a substituent to be a ‘deactivating’ meta director?
Needs to be electron withdrawing, eg NO2
How do halogens behave as a substituent on benzene?
They withdraw electron density by induction (electronegative) but they donate density via conjugation (their lone pairs).
They deactivate the ring, but are ortho para directors.
Cl, Br and I have poor orbital overlap however, meaning the degree of lone pair conjugation with the ring is limited (less reactive than a NH2, for example).
What is the mechanism for the nucleophilic aromatic substitution of benzene?
The electron withdrawing group needs to be in either the ortho or para position.
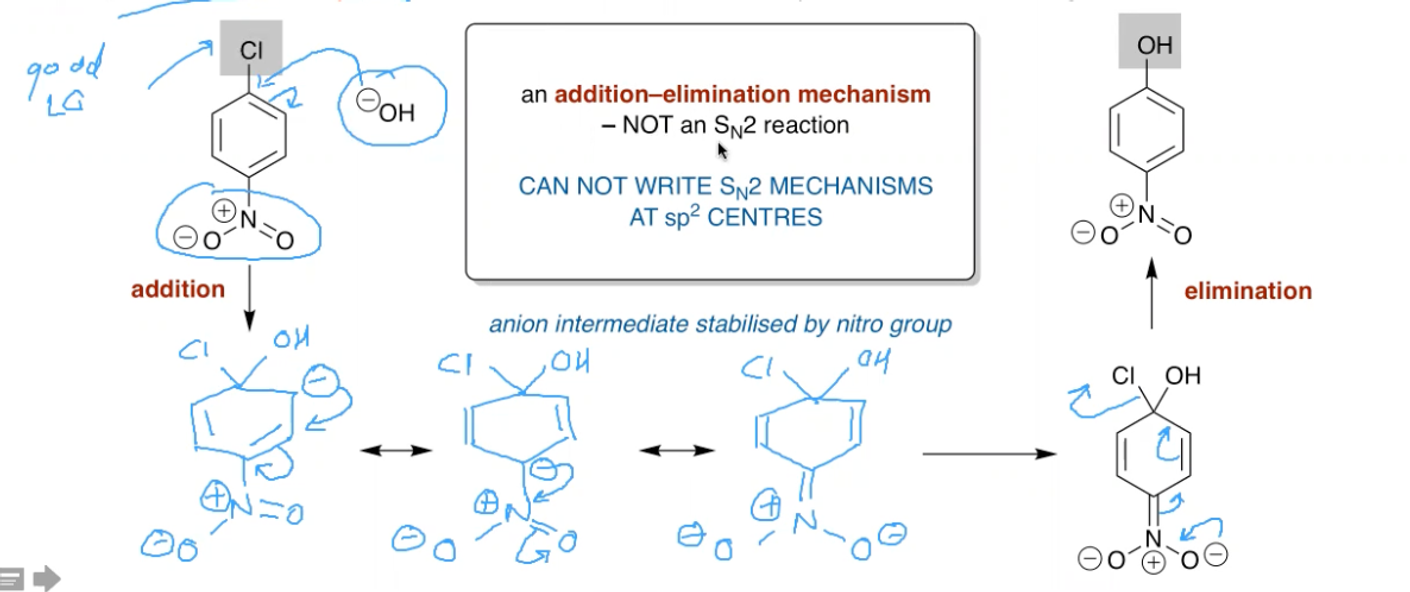
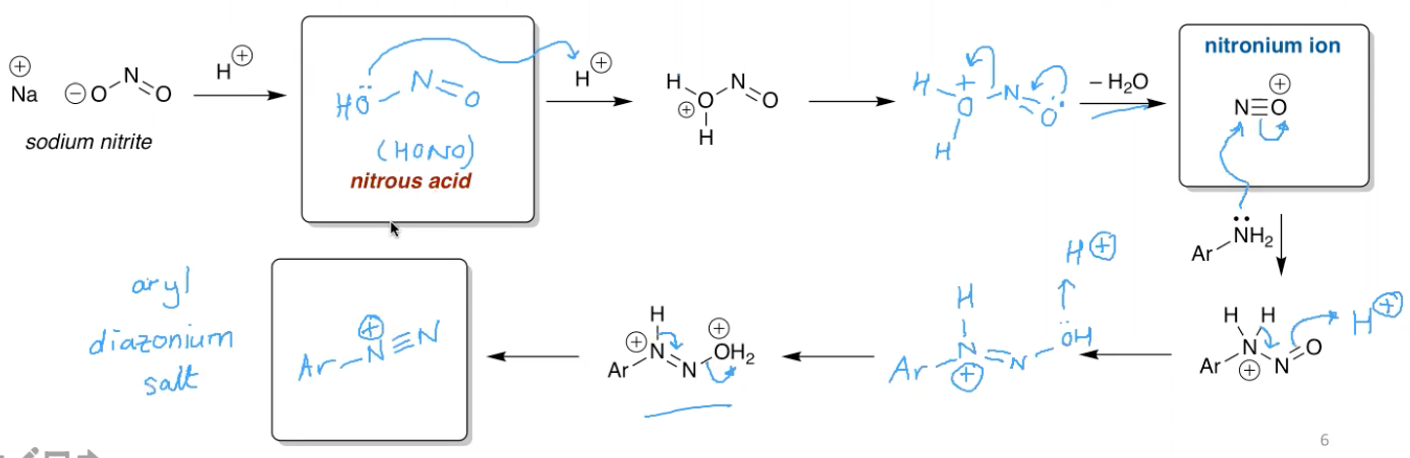
What is the mechanism for the formation of an aryl diazonium salt? (off NH2)
Uses NaNO2 and HCl
What is the use of having an aryl diazonium salt?
You can perform Sandmeyer reactions, which are the copper mediated manipulator of aryldiazonium salts.
You can also reduce them, removing the N2 group entirely. This allows for some techy manoeuvres where you can nitrate a benzene ring using the NO2 as a meta director, then convert it to a AD salt, then reduce the salt after the reaction has been completed.
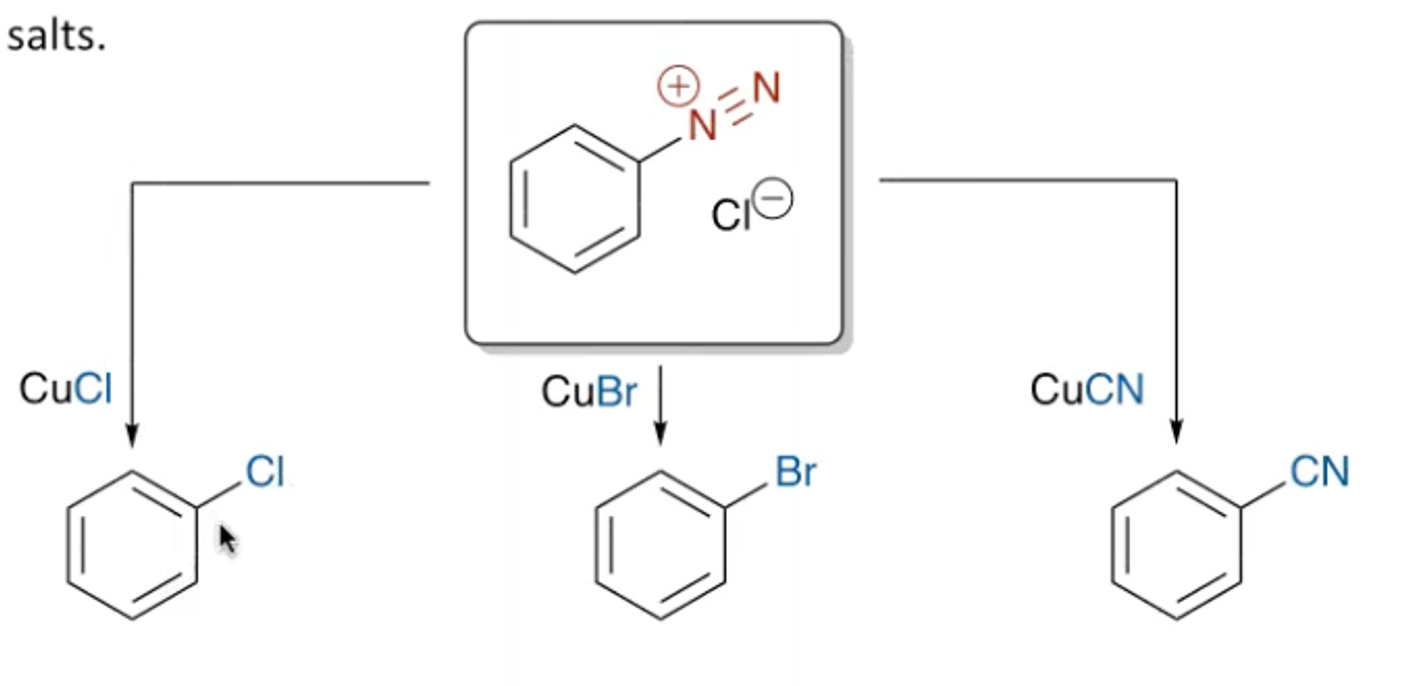
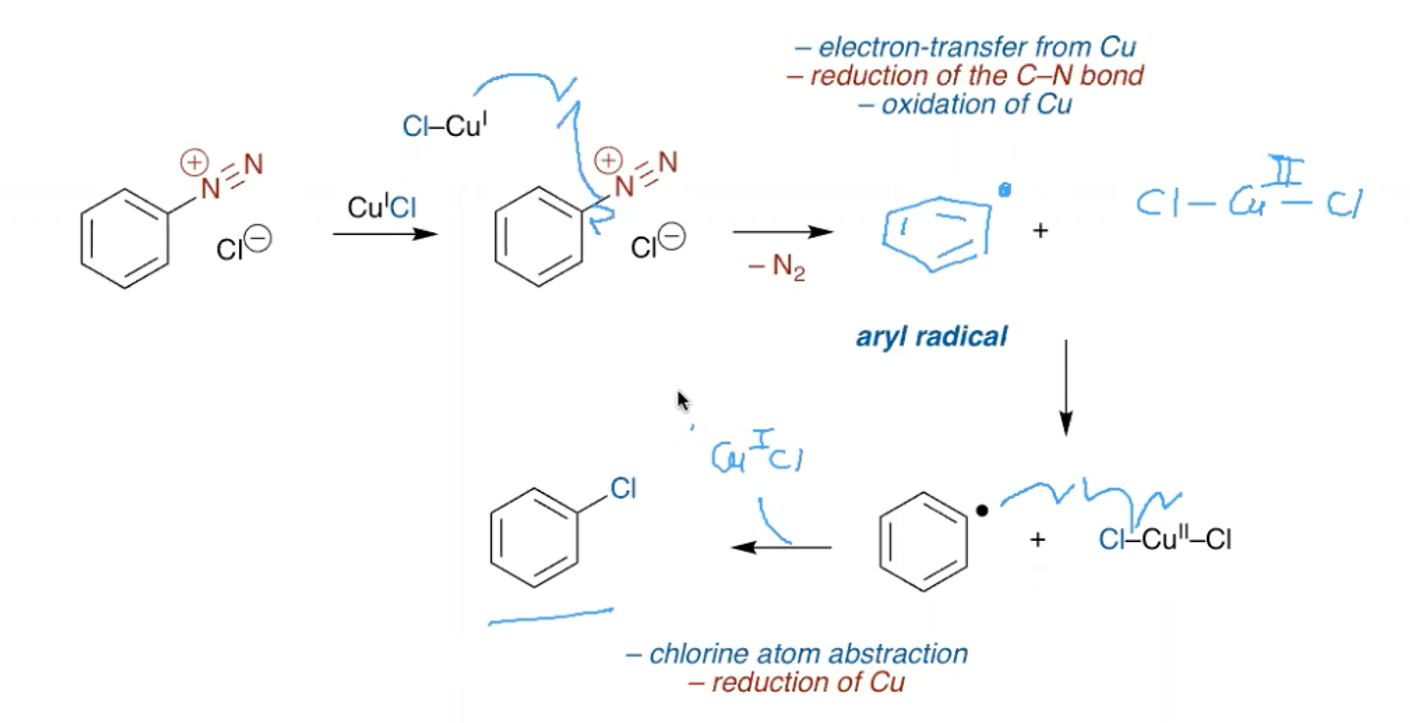
What is the mechanism for the Sandmeyer reactions?
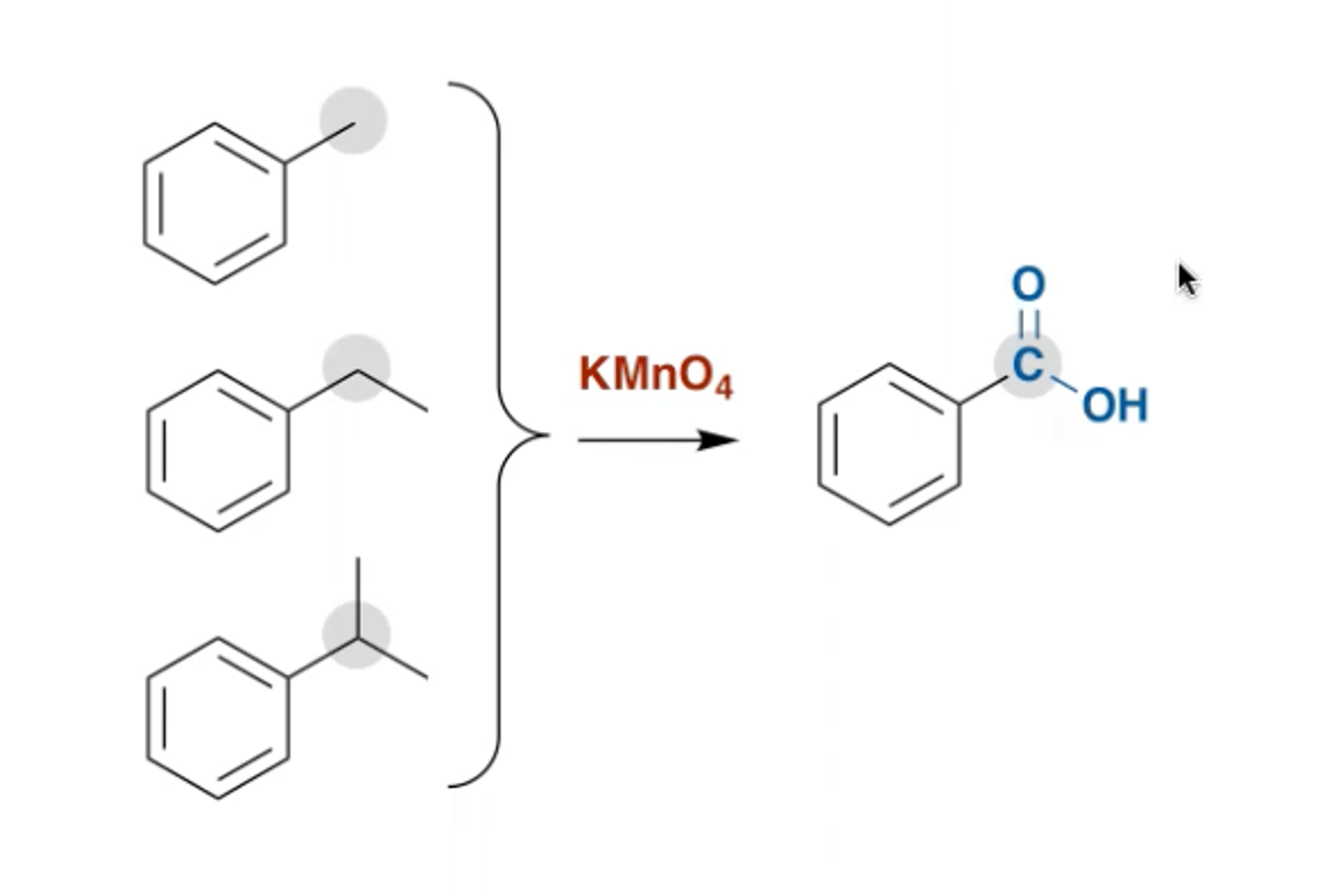
How can you oxidise the alkyl group attached to a benzene ring? What does it form?