CHEM 1411 Exam 3 Review
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
39 Terms
Hund’s Rule
Each orbital within a set of degenerate orbitals is occupied by a single electron before being doubly occupied.
Octet Rule
The main group elements tend to form bonds to have 8 valence electrons.
The Aufbau Principle
Electrons are arranged in an atom by building up from the ground state.
Pauli Exclusion Principle
No two electrons in the same atom may have identical sets of the four quantum numbers.
The Heisenberg Uncertainty Principle
It is impossible to know the exact position and momentum of a particle simultaneously.
How do you determine i
Photon
a particle of light; packet of electromagnetic energy. used to carry energy that can excite electrons or cause transitions.
Valence Electrons
electrons in the outermost shell. used in bonding and chemical reactions.
Core Electrons
electrons in the inner shells, not involved in bonding. help shield valence electrons.
Isoelectronic
atoms or ions with the same number of electrons. ex: Na+,Ne, and F- all are isoelectronic (10 electrons).
Ionic Compound
A compound that consist of a metal and a nonmetal, involves electron transfer
Covalent Compound
A compound made of only nonmetals, involves sharing electrons.
Nonpolar Covalent Bond/Compound
electrons are shared equally between atoms. happens when atoms have similar electronegativities.
Polar Covalent Bond/Compound
electrons are shared unequally, creating a dipole. one atom pulls electrons morel causing asymmetrical polarity.
Electron Deficient
atoms that have fewer than 8 electrons in their valence shell common in Boron compounds
Hypervalent
atoms that have more than 8 electrons in their valence shell. only found in elements in period 3 or higher.
Electronegativity
an atom’s ability to attract electrons in a bond, determines bond polarity; F is the most electronegative
Formal Charge
the charge assigned to an atom in a molecule assuming equal sharing. formal charge = valence electrons - (lone pairs + ½ bonding electrons)
n
represents the principal quantum number, indicating the energy level of an electron in an atom.
l
Angular momentum quantum number; tells you the shape of the orbital and the subshell type (s, p, d, f) within a given energy level. From 0 to n-1
m1
Magnetic quantum number; indicates the orientation of the orbital in space. It can have values from -l to +l.
m2
Spin quantum number; describes the intrinsic angular momentum of an electron, with possible values of +1/2 or -1/2.
Which orbital would be represented by an angular momentum number of 0?
Orbital “s”
Which orbital would be represented by an angular momentum number of 1?
Orbital "p"
Which orbital would be represented by an angular momentum number of 2?
Orbital “d”
Which orbital would be represented by an angular momentum number of 3?
Orbital "f"
How does atomic radius trend?
Increasing going down and leftward with the greatest being Francium.
How does electronegativity trend?
Increasing going up and rightward with the greatest being Fluorine.
How does ionization energy trend?
Increasing going up and rightward with the greatest being Helium.
How does charge affect atomic radius?
A negative charge indicates that the radius has increased and a positive indicates a decrease.
If you were to have multiple ions with the same number of electrons, how would you be able to tell which element has the greatest atomic radius?
The ion with the least number of protons would have the greatest atomic radius.
When writing electronic configurations where do the d levels fall?
After the next shells s. (example 4p6 5s2 4d10)
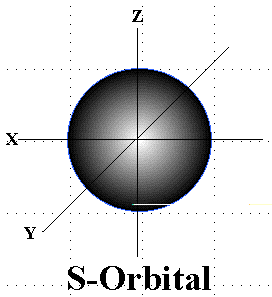
What is the shape of the “s” orbital?
The shape of the "s" orbital is spherical, centered around the nucleus.
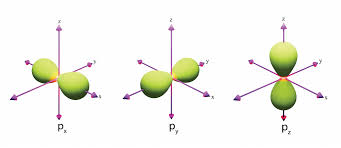
What is the shape of the “p” orbital?
The shape of the "p" orbital is dumbbell-shaped, oriented along three axes (x, y, and z) in space.
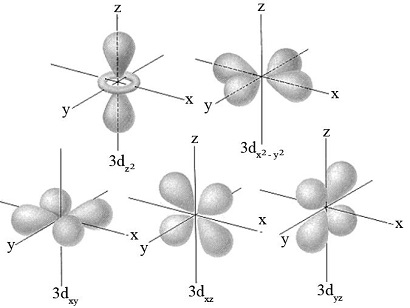
What is the shape of the “d” orbital?
The shape of the "d" orbital is complex and can vary, but typically includes cloverleaf patterns and a doughnut-shaped ring around the center. There are five different d orbitals corresponding to different orientations in space.
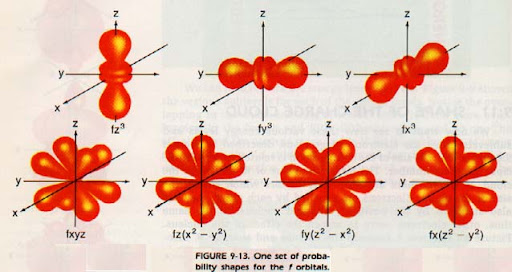
What is the shape of the “f” orbital?
The shape of the "f" orbital is even more complex than that of the "d" orbitals, characterized by multiple lobes and intricate patterns. There are seven different f orbitals, each with unique spatial orientations.