6. modes of inheritance II
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
atypical inheritance
Mosaicism
Dynamic mutations: unstable repeats
Mitochondrial disorders
Mosaicism
Individual with at least 2 cell lines that differ genetically, but are derived from a single zygote
Mutations that occur after conception in a single cell during either pre/postnatal life are producing clonal descendants that are genetically different from the original zygote
Whether mosaicism involves germline cells, somatic cells, or both
depends on:
the time the mutation has occurred during embryogenesis
Somatic mosaicism
Mutation that occurs during embryogenesis and affects morphogenesis → segmental abnormality depending on the stage when the mutation occurred and the lineage of the cells affected
Mutation in adult dividing cells → carcinogenesis
Germline mosaicism
Unaffected individuals with no evidence of disease-causing mutation (not detected in the DNA from buccal swabs or peripheral blood cells)
may be at risk of having offspring with highly penetrant autosomal dominant or X-linked disease
Dynamic mutations: unstable repeats
In all other types of inheritance, a mutation is stable through generations
A class of hereditary disease (mostly neurological) are due to unstable repeat expansions = expansion of a DNA segment consisting of repeating units of nucleotides
Various patterns of inheritance
Polyglutamine disorders
Huntington’s disease
string of consecutive Glu (CAG) of variable length in the mutant protein
characteristics of poilyglutamine disorders
Follows inheritance pattern similar to autosomal dominant (50% risk) with
a few specific characteristics
Anticipation = earlier onset with each generation
Repeat expansion (nr. of repeats) dictates the severity of symptoms
parental transmission bias
parental transmission bias
Anticipation and repeat expansion more severe when inherited from father
normal vs a lot of repeats → age of onset + severity for HD
Normal number of repeats = 5-35; mild HD with late onset
36-39; severe HD >40 repeats
Fragile X syndrome
most heritable form of mild intellectual disability
X-linked dominant inheritance with variable expressivity and low penetrance (50% in females)
Disease caused by massive repeat expansion (>200 CGG) in 5’-UTR of FMR1 gene → increased methylation on CpGs (constriction of the region under the microscope) and gene silencing
Mitochondrial disorders
Do NOT follow mendelian inheritance, strict maternal inheritance
Each cell contains several hundred mitochondria, and each mitochondria
has several copies of mtDNA (mtDNA has no introns)
>100 rearrangements and point mutations (increased oxidative stress
and lack of repair mechanisms) in the mtDNA → increased pleiotropy
Patterns of mitochondrial inheritance result from the specific features of mitochondrial biology
1. Maternal inheritance
2. Replicative segregation
3. Homoplasmy/heteroplasmy
Patterns of mitochondrial inheritance result from the
specific features of mitochondrial biology: 1. Maternal inheritance
Sperm mitochondria is not present in the zygote
Patterns of mitochondrial inheritance result from the
specific features of mitochondrial biology: 2. Replicative segregation
During cell division mtDNA replicates and copies are distributed randomly among newly synthesized mitochondria → significant variability in disease manifestation among tissues and individuals
Patterns of mitochondrial inheritance result from the
specific features of mitochondrial biology: 3. Homoplasmy/heteroplasmy
De novo mutations appear in only one of the mtDNA in one mitochondrion, but mtDNA replication produces multiple mutant mtDNA
Replicative segregation accounts for the random distribution of mutant and wt mitochondria to daughter cells
homoplasmy
Pure population of either wt or mutant mtDNA
heteroplasmy
Mixed population
Phenotypic expression depends on:
the relative proportions of wt and mutant mtDNA in the cells of a tissue → low penetrance and high expressivity of mitochondrial disorders
mitochondrial genetic bottleneck
the number of mitochondria in developing oocytes is reduced before
expansion to the number seen in mature oocyte
leads to variability in proportion of mutant mtDNA in offspring
multifactorial inheritance
everything below
Multifactorial diseases
complex inheritance
Caused by additive effects of multiple genetic variants (polygenic) and environmental factors
Classification:
– Discrete qualitative traits (either present or not)
– Continuous quantitative traits
complex inheritance
Familial clustering, but NOT in a mendelian pattern
quantitative traits
measurable physiological or biochemical parameters that vary among individuals
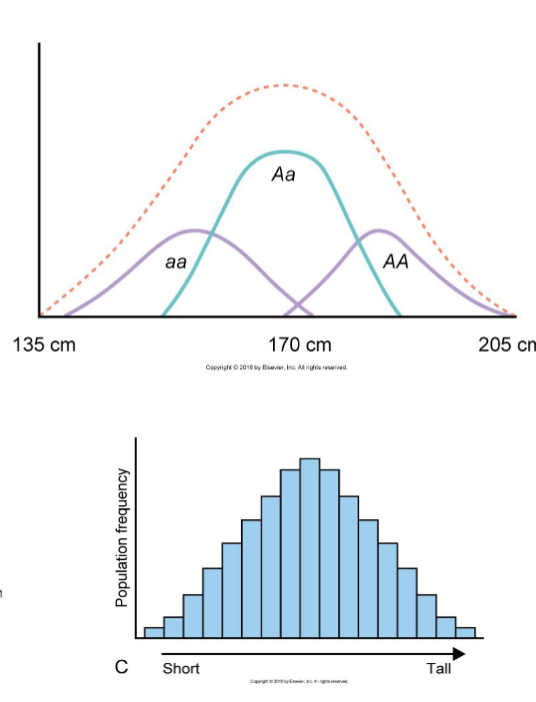
Trait distribution of quantitative traits
Most quantitative traits follow a normal (gaussian)
distribution
– eg. >200 loci associated with height
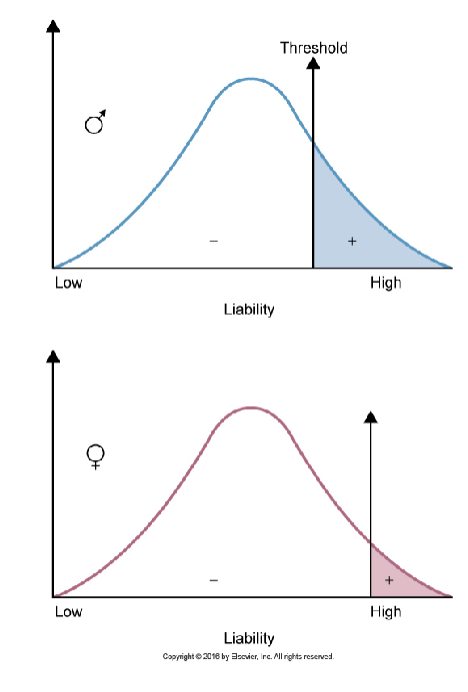
Qualitative traits distribution
have a liability distribution
the threshold of liability must be crossed for an individual to express the disease
Criteria for multifactorial inheritance
Difficult to determine recurrence risk, therefore empirical risk is calculated based on studies of large number of affected families
Recurrence risk is higher if:
>1 family member is affected
the proband has a severe form of disease
the proband is of the less commonly affected sex
Recurrence risk decreases rapidly in distant relatives
the empirical risk varies drastically between populations due to
variability in allele frequency and environmental factors
Relative contributions of genes and environment: 1. Twin studies
Differences in monozygotic twins (MZ) due ONLY to environmental factors (dizygotic twins (DZ) serve as comparison)
concordant for the trait
If both twins have same disease
if not, discordant
Concordance
Concordance < 100% in monozygotic twins → non-genetic factors at play
The greater the concordance in MZ vs. DZ, the stronger the genetic component
Concordance rates and correlations are used to measure heritability
heritability
proportion of phenotypic variation of a trait that is due to underlying genetic variation
Heritability values are specific for the population in which they
were estimated
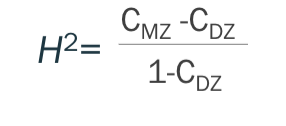
Limitations to twin studies
Assumption of equally similar environments for MZ and DZ (MZ twins raised separately would be perfect controls)
Different somatic mutations after cleavage
Methylation patterns (and X-inactivation patterns in females)
Relative contributions of genes and environment: 2. Adoption studies
Comparison of disease rates among adopted offspring of affected parents with the rates of adopted offspring of unaffected parents → establish genetic component
Limitations to adoption studies
Non-random adoption process
Congenital malformation examples
Congenital heart defects + Cleft-lip and palate
cardiovascular disorder examples
coronary artery disease, stroke, hypertension
cancer examples
breast, colorectal, + prostate
diabetes examples
T1DM + T2DM
other multifactorial disease examples
obesity, alzheimers, alcoholism
Neuropsychiatric disorder examples
autism, schizophrenia