Chapter 7: Atmospheric Pollution (copy)
1/95
Earn XP
Description and Tags
Environmental Science
Pollution
AP Environmental Science
Atmospheric Pollution
Air Pollution
Atmospheric CO2 and Particulates
Lead
Nitrogen Oxides
Carbon Monoxide
Ozone
Peroxyacyl Nitrates
Sulfur Dioxides
Suspended Particulate Matter
Volcanic Organic Compounds
Thermal Inversion
Photochemical Smog
Air Pollutants
Acid Rain
Deposition
Noise Pollution
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
96 Terms
Antarctica
________ has a nearly constant temperature inversion.
Air pollution
It occurs when harmful or excessive quantities of substances are introduced into Earths atmosphere
Parts per million (ppm)
The most common form of expressing air pollutants
Primary Pollutants
Emitted directly into the air
Examples of primary pollutants
CO, SO2, Particulate matter (PM)
Secondary Pollutants
Result from primary air pollutants reacting together and forming new pollutants
Examples of secondary pollutants
Ozone (O3), NO2, H2SO4
What are 3 main anthropogenic sources of gaseous air pollution?
Vehicle Emissions: Combustion engines emit pollutants like carbon monoxide, nitrogen oxides, and hydrocarbons
Industrial Processes: Factories release pollutants such as sulfur dioxide, Nitrogen oxides, and PM during production
Agricultural activities: Livestock farming and fertilizer application release pollutants like ammonia and methane into the air
Primary pollutants from coal combustion
Sulfur Dioxide
Nitrogen Oxide (NOx)
Particulate Matter (PM)
Carbon Monoxide (CO)
Mercury (HG)
VOCs
Primary pollutants from motor vehicle exhaust
Nitrogen oxides (NOx)
Carbon Monoxide (CO)
Hydrocarbons (HC)
Particulate matter (PM)
Lead (Pb)
Point source air pollution
It occurs when the contaminant comes from an obvious source
Non-point source air pollution
It occurs when the contaminant comes from a source that is not easily identifiable or from a number of sources spread over a large, widespread area
Clean Air Act
identified 6 criteria air pollutants that the EPA is required to set acceptable limits for, monitor, and enforce
Provisions of the Clean Air Act
Sets national air quality standards
Regulates emissions of hazardous Air pollutants
Requires permits for major sources of air pollution
Establishes regulations for vehicle emissions
How does Cap and trade relate to the clean air act?
Cap and trade, a market-based approach, allows regulated entities to trade emissions allowances. The Clean Air Act enables the implementation and regulation of such programs to reduce air pollution.
Criteria air pollutants
These are a set of eight air pollutants that cause smog, acid rain, and other health hazards and are typically emitted from many sources in the industry, mining, transportation, power generation, and agriculture
The Eight Criteria Air Pollutants
NOSCLP (Nose clip)
Nitrogen Oxides, Ozone, Sulfur Dioxide, Carbon Monoxide, Lead, and Particulate matter
Industrial smog
Trends to be sulfur-based and is also called gray smog
Carbon monoxide
It is a colorless, odorless, and tasteless gas that is slightly less dense than air and is produced from the partial oxidation of carbon-containing compounds
Carbon monoxide effects
Bonds to hemogoblin, thereby interfering with oxygen transport in the blood stream
Death with prolonged exposure at high concentrations
Headaches, dizziness, nausea, fatigue, death
Sources of Carbon Monoxide
Incomplete combustion of any kind
Malfunctioning exhaust systems and poorly ventilated cooking fires
Lead
It is used in building construction, lead-acid batteries for vehicles, bullets and shot fishing weights, solder, and shields for radiation
Lead Effects
Impairs central nervous system, affects learning and concentration
Neurological damage, developmental issues, organ damage
Sources of lead
Gasoline additive
Oil and gasoline
coal
old paint
Anthropogenic
Nitrogen Oxides (NOx)
A generic term for nitric oxide and nitrogen dioxide, which are produced from the reaction of nitrogen and oxygen gases in the air
Nitrogen oxide effects
Respiratory irritant, precursor ozone, leads to photochemical smog, creates nitric acid, over fertilizes land and water
Soil acidification
water pollution
eutrophication of lakes and rivers
harm to aquatic life
Respiratory issues, exacerbates asthma
Sources of nitrogen oxides
All combustion in the atmosphere including FF combustion, wood, and other biomass burning
Anthropogenic: Vehicle emissions, FF combustions
Anthropogenic, Combustion
Nitrous oxide
It is a major air pollutant, with levels of N2O having increased by more than 15% since 1750
Ozone
It is an inorganic molecule with the chemical formula O3, and tropospheric (ground-level) ozone is a secondary air pollutant
Ozone Effects
Reduces lung function and exacerbates respiratory symptoms
Degrades plant surfaces
Damages materials such as rubber and plastic
Cause asthma and bronchitis
Harm lung function and irritate the respiratory system
Result in heart attacks and other cardiopulmonary problems
Suppress the immune system.
Sources of OZONE
Secondary pollutant formed by the combination of sunlight, water, oxygen, VOCS, and NOx
Anthropogenic sources: Vehicle emissions, industrial processes
Tropospheric ozone
It does not have strong global effects, but instead is more influential in its effects on smaller, more localized areas
Peroxyacyl Nitrates (PANs)
These are secondary pollutants
Sulfuric Dioxide
A colorless gas with a penetrating, choking odor that readily dissolves in water to form an acidic solution
Effects of Sulfur Dioxide
Respiratory irritant, Can exacerbate asthma and other respiratory ailments
Harm stomata and plant tissue
In atmosphere - harmful to aquatic life
Soil acidification
water pollution
damage to building and monuments
Respiratory issues, cardiovascular problems
Sources of sulfur dioxide
combustion of coal, oil, and gasoline
Anthropogenic: Industrial processes, Combustion of FFs, transportation
Anthropogenic, Combustion
Particulate matter (PM-2.5 PM2.10)
Sources: Combustion of coal, oil, diesel,
biofuels: agriculture, road construction
Suspended particulate matter (PMx)
It is microscopic solid or liquid matter suspended in Earths atmosphere
Volatile Organic Compounds (VOCs)
Air pollutant
These are organic chemicals that have a high vapor pressure (easily evaporate) at ordinary room temperature
Sources of VOC
Evaporation of fuels, paints, incomplete combustion of fuels
Anthropogenic
Effects of VOC
Precursor to OZONE formation
Eye, nose, and throat irritation, headaches, nausea
Mercury (Hg)
Air pollutant
Sources: Coal, oil, mining
Effects: Impairs Central nervous system, bioaccumulates in the food chain
Carbon Dioxide (CO2)
Sources: Combustion of Fossil Fuels and clearing of land
Effects: Affects climate and alters ecosystems by increasing green house gas emissions
Hydrocarbons
Pollutant compounds that contain carbon, hydrogen bonds such as
gasoline and other FF
lighter fluid
Dry cleaning fluid
oil based plants
perfumes
OZONE (O3) Photochemical Smog
Primary pollutant (O3)
Anthropogenic sources: Vehicle emissions, industrial processes
Secondly pollutants: Nitrogen dioxide (NO2), peroxyacetyl nitrate (PAN)
Effects on human health: Irritation of eyes, nose, and throat; respiratory issues
Nitrogen Dioxide (NO2) Photochemical smog
Primary Pollutant: NO2
Anthropogenic sources: Vehicle emissions, fossil fuel combustion
Secondary pollutants: Ozone (O3), nitric acid (HNO3)
Effects on human health: Respiratory problems, lung damage, increased susceptibility to respiratory infections
Volatile Organic Compounds (VOCs)-
Primary pollutant (VOCs)
Anthropogenic sources: Vehicle emissions, industrial processes
Secondary pollutants: Ozone (O3), formaldehyde, acetaldehyde
Effects on human health: Eye, nose, and throat irritation; headache; liver and kidney damage
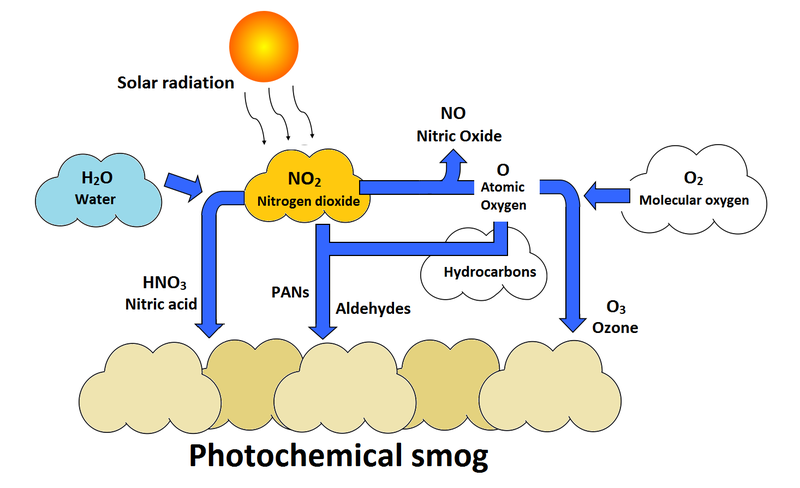
Photochemical smog (LA Smog, brown smog)
It is catalyzed by ultraviolet (UV) radiation, tends to be nitrogen-based,
Categorized by oxidants such as ozone
Effects of Photochemical smog on Human Health
Irritation of respiratory system
aggravation of asthma and other respiratory diseases
increased risk of cardiovascular diseases
Three ways to reduce Photochemical smog
Implement stricter standards for vehicles and industries
encourage alternate transportation methods
promote adoption of cleaner technologies and renewable energy sources
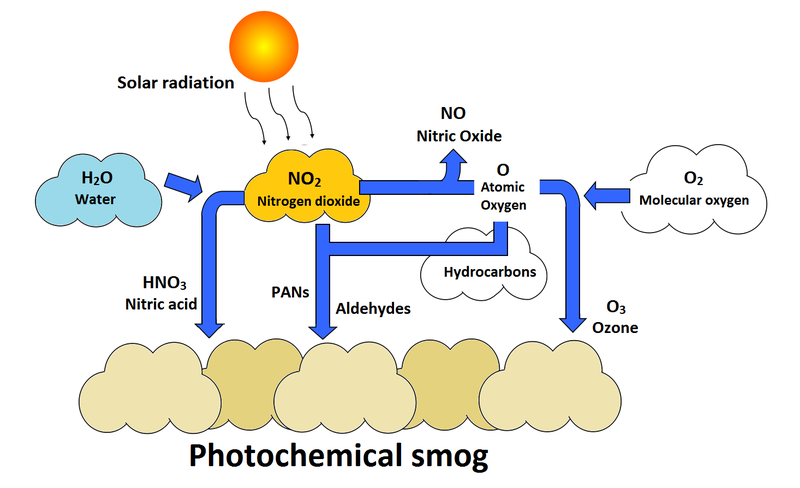
Formation of Photochemical smog
Picture
Sulfurous smog (London smog, gray smog, industrial smog)
smog dominated by sulfur dioxide, sulfate compounds, and Particulate matter
Natural formation and destruction of ozone (ABSENCE OF VOCs)
in the absence of VOCs Ozone will form during the daylight hours
After sunset, the ozone will break down
Formation of photochemical smog (VOCs Present)
In the presence of VOCs, Ozone will form during the daylight hours. The VOCs combine with nitrogen oxides to form photochemical oxidants, which reduce the amount of ozone that will break down later and contribute to prolonged period of photochemical smog
How ground level ozone forms
Ground-level ozone forms through a chemical reaction involving nitrogen oxides (NOx) and volatile organic compounds (VOCs) in the presence of sunlight, warm temperatures, and still air conditions.
The main product of this reaction is ozone (O3)
What accelerates the formation of ground level ozone
vehicle emissions, industrial processes, and fossil fuel combustion are primary sources that accelerate the formation of ground-level ozone.
Measures to Reduce Formation ground level ozone
stricter emission standards for vehicles and industries
Cleaner technologies
renewable energy sources
alternative transportation methods
Ground level ozone effects on human health
irritation of the eyes, nose, and throat,
respiratory problems such as coughing and wheezing
aggravation of asthma and other respiratory diseases
increased susceptibility to respiratory infections.
Thermal inversions
These occur when air temperature rises with height instead of falling
two things that can be done to help mitigate the effects of thermal inversions
Reducing Emissions: Implementing measures to reduce emissions of air pollutants from industrial sources, vehicles, and other anthropogenic activities can help minimize the formation of pollutants trapped during thermal inversions.
Urban Planning: Implementing urban planning strategies such as increasing green spaces, reducing the concentration of buildings, and promoting sustainable transportation options can help disperse pollutants and alleviate the impacts of thermal inversions.
Human Health Effects of Thermal Inversions
Respiratory Problems: Thermal inversions can lead to the accumulation of pollutants near ground level, exacerbating respiratory issues such as asthma, bronchitis, and allergies due to increased exposure to harmful air pollutants.
Cardiovascular Issues: Prolonged exposure to pollutants trapped during thermal inversions can also increase the risk of cardiovascular diseases, including heart attacks and strokes, particularly in vulnerable populations such as the elderly and individuals with pre-existing heart conditions.
"Sick building" syndrome (SBS)
It is a term used to describe a combination of ailments associated with an individuals place of work or residence
Asbestos
It is inexpensive, durable, and flexible and naturally acts as an insulating and fireproofing agent
Carbon monoxide poisoning
It is the most common type of fatal indoor air poisoning in many countries because it easily combines with hemoglobin to block the bloods oxygen-carrying capacity
Formaldehyde
It is an organic chemical that is prevalent in the indoor environment and is a carcinogen that is linked to lung cancer
Sources: Anthropogenic
Health issues: Eye, nose, and throat irritation, respiratory issues
Radon-222
It is an invisible radioactive gas that results from the radioactive decay of radium, which can be found in rock formations beneath buildings
Source: Natural
Lung cancer, respiratory issues
Two ways to prevent radon-222 from entering homes are
Sealing foundation cracks and openings in walls or floors.
Installing a radon mitigation system, such as a vent pipe and fan system.
Which areas of the world have the most problems with air pollution?
densely populated urban areas and regions with high levels of industrial activity.
Conversely, regions with the most outdoor air pollution issues often include urban centers with heavy traffic, industrial activity, and geographical features that trap pollutants, such as valleys or basins.
Cigarette smoke
It contains almost 5,000 chemical compounds, including 60 known carcinogens (cancer-causing chemicals), one of which is dioxin
Anthropogenic
Respiratory issues, cancer, heart disease
Wet and Dry Scrubbers
pollution control devices used to remove pollutants from industrial exhaust gases. Wet Scrubbers Uses liquid to trap pollutants, while dry scrubbers use solid material or chemicals for absorption or adsorption.
Targeted Pollutant: SO2, PM
Source of Pollutant: Industrial emissions
Practice type: Regulatory
Boghouse filters
wastewater treatment systems that use layers of media to filter and treat sewage through biological and physical processes, improving water quality.
Targeted pollutant: PM
Source of pollutant: industrial emissions
Practice type: regulatory
Fluidized bed combustion
combustion technology where solid particles are suspended and heated in a fluidized state, enhancing combustion efficiency and reducing emissions in processes like power generation and waste incineration.
Targeted pollutant: SO2, NOx
Source of pollutant: power plants, industrial boilers
Practice type: Conservation
Electrostatic precipitators
emission control devices that use electrostatic forces to remove particles and pollutants from exhaust gases in industries such as power plants and steel mills.
Targeted pollutant: PM
Source of pollutant: Power plants, industrial boilers
Practice type: Regulatory
Catalytic converter
Reduction Method
It is an exhaust emission control device that converts toxic chemicals in the exhaust of an internal-combustion engine into less harmful substances
Practice type: regulatory
Target pollutant: Nix, CO, HC
Source of pollutant: Automobile emissions
Vapor Recovery Nozzle
Reduction method Device designed to capture and contain gasoline vapors during refueling, reducing emissions and environmental impact.
Target pollutant: VOCs
Source of Pollutant: Automobile Emissions
Practice type: Regulatory
Catalyst
It stimulates a chemical reaction in which by-products of combustion are converted to less toxic substances by way of catalyzed chemical reactions
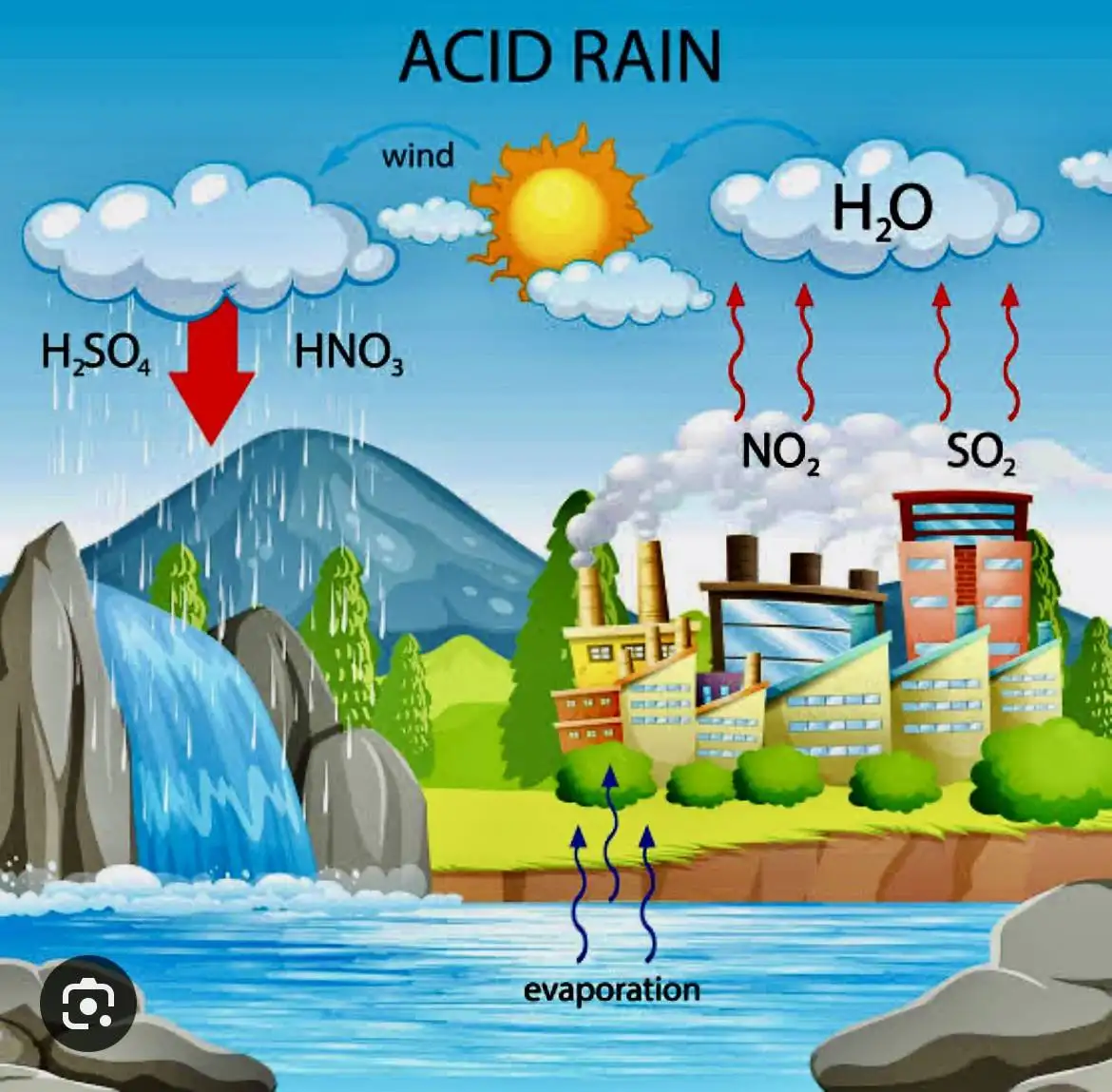
Acid deposition
It occurs when atmospheric chemical processes transform sulfur and nitrogen compounds and other substances into wet or dry deposits on Earth
Dry Deposition
In dry areas, acidic chemicals in the air may become dust or smoke and stick to the ground, buildings, homes, cars, and trees, which rainstorms wash away, increasing acidic runoff
Wet Deposition
Acid rain, fog, and snow
Acid rain
It causes acidification of lakes and streams
Primary pollutants: Sulfur Dioxide (SO2), Nitrogen Oxides (NOx)
Anthropogenic sources: Industrial emissions, transportation, combustion
Secondary Pollutant(s): Sulfuric Acid (H2SO4), Nitric Acid (HNO3)
Most Affected Areas: North America, Europe, Asia
Effects on Human Health/Ecosystems: Respiratory issues, soil acidification, aquatic ecosystem damage
Three ways to reduce acid rain formation
Implementing stricter emissions regulations for industrial and transportation sectors.
Promoting the use of cleaner energy sources such as renewable energy and natural gas.
Installing pollution control technologies like scrubbers in industrial facilities.
Effects of acid rain
Soil: Acidification leading to nutrient depletion and decreased plant growth.
Water: Acidification of lakes, rivers, and streams, harming aquatic life and disrupting ecosystems.
Plants: Damage to leaves and needles, reduced photosynthesis, and inhibited growth.
Outdoor structures: Corrosion and deterioration of buildings, monuments, and infrastructure.
Acid Rain Formation
picture
Acid shock
Caused by rapid melting of snow pack with dry acidic particles, raises lake and stream acid concentrations five to ten times higher than acidic rainfall
Urban heat islands
It occur in metropolitan areas that are significantly warmer than their surroundings
Street Canyon
A place where the street is flanked by buildings on both sides, creating a canyon-like environment
Noise pollution
It is an unwanted human-created sound that disrupts the environment
Transportation
Industrial activities
Human actives (recreational, events, etc)
Urban development (Roads & bridges)
Ecological effects of Noise pollution on animals
Behavioral changes: disruption of natural behaviors; feeding, mating, and communication
Habitat displacement: Drive animals away from their habitats, leading to a loss of territory and resources
Stress and health issues:
Sensory hearing loss
it is caused by damage to the inner ear and is the most common form associated with noise pollution.
sulfur dioxide
Acid deposition due to ______ begins with sulfur dioxide being introduced into the atmosphere by burning coal and oil, smelting metals, organic decay, and ocean spray.
6 A.M.–9 A.M.
As people drive to work, concentrations of nitrogen oxides and VOCs increase
9 A.M.–11 A.M.
As traffic begins to decrease, nitrogen oxides and VOCs begin to react, forming nitrogen dioxide (NO2)
11 P.M.–4 P.M.
As the sunlight becomes more intense, nitrogen dioxide is broken down and the concentration of ozone (O3) increases:
4 P.M.–Sunset
As the sun goes down, the production of ozone is halted.
three way
Most present-day vehicles that run on gasoline are fitted with a “______” converter, since it converts the three main pollutants:
Carbon cycle
Photosynthesis: Naturally occurring process where plants absorb carbon dioxide (CO2) from the atmosphere and convert it into organic compounds, releasing oxygen (O2) as a byproduct.
Cellular Respiration: Both naturally occurring and anthropogenic, where organisms break down organic compounds to release energy, producing CO2 as a byproduct.
Decomposition: Naturally occurring process where dead organisms and organic matter decay, releasing CO2 back into the atmosphere.
Combustion: Anthropogenic process involving the burning of fossil fuels, biomass, and other organic materials, releasing CO2 into the atmosphere.
Volcanism: Naturally occurring process where volcanoes release CO2 and other gases during eruptions.
Three sources of naturally occurring particulate matter include volcanic eruptions, wildfires, and dust storms.