chemistry - key concepts in chemistry: covalent bonding (1.28 - 1.31)
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
1.28 how are covalent bonds formed?
pairs of electrons shared between 2 non-metal atoms
strong covalent bond - strong electrostatic forces of attraction between bonded atoms
weak intermolecular forces - weak forces of attraction between molecules
1.29 what does covalent bonding form?
molecules
1.30 typical size of atoms & small molecules
diameter ≈ 10-10m
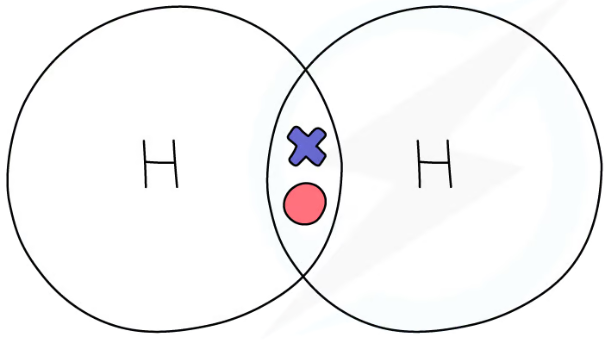
1.31 simple molecular, covalent substances dot & cross diagrams - hydrogen
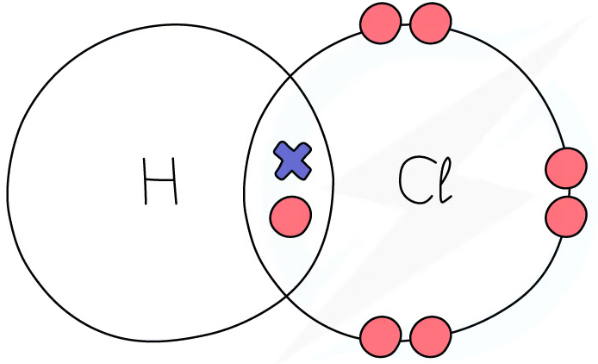
1.31 simple molecular, covalent substances dot & cross diagrams - hydrogen chloride
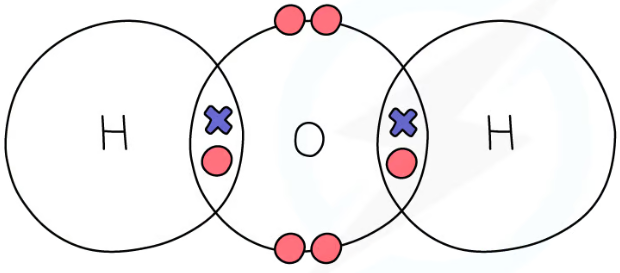
1.31 simple molecular, covalent substances dot & cross diagrams - water
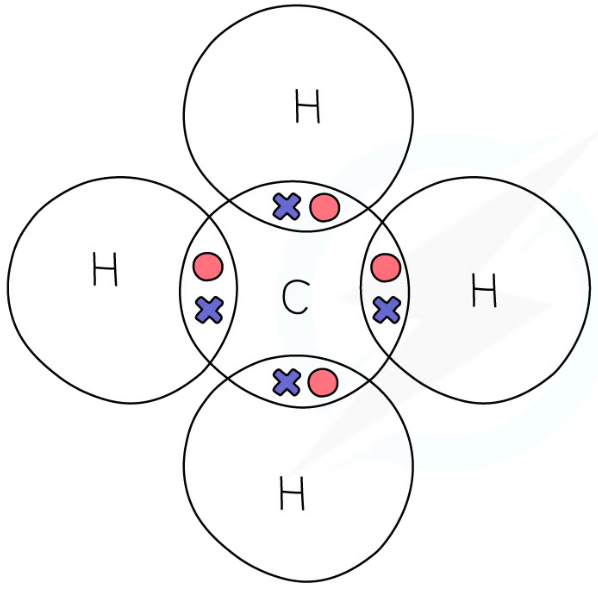
1.31 simple molecular, covalent substances dot & cross diagrams - methane
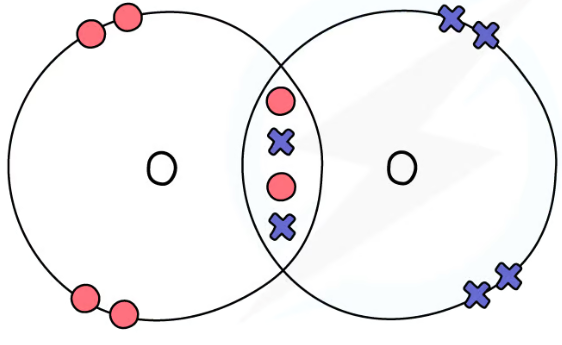
1.31 simple molecular, covalent substances dot & cross diagrams - oxygen
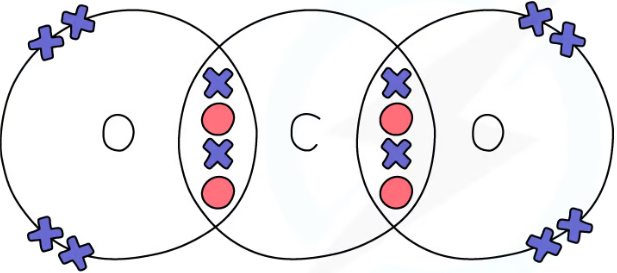
1.31 simple molecular, covalent substances dot & cross diagrams - carbon dioxide