X-Ray Photoelectron spectroscopy
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
lonization Energy
Energy required to remove an electron to an infinite distance at rest from an atom, molecule, or ion.
Photoelectric Effect
Observation that a minimum frequency (energy) of light was needed to remove any electrons (photemmision), minimum energy corresponds to the work function o of the material.
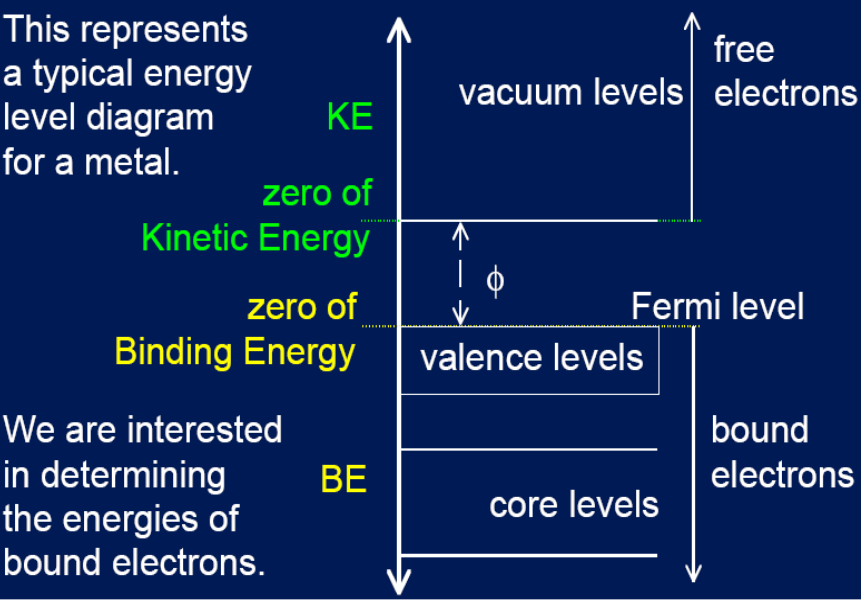
Metal energy diagram
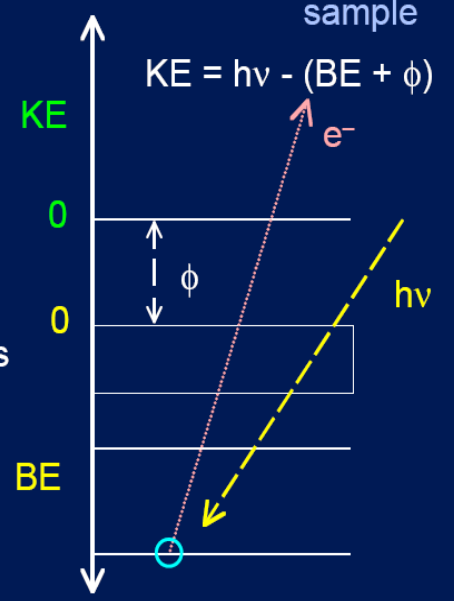
Photoemmision Event
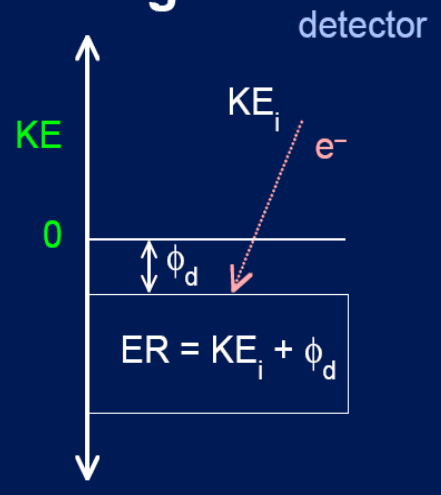
Detection Event
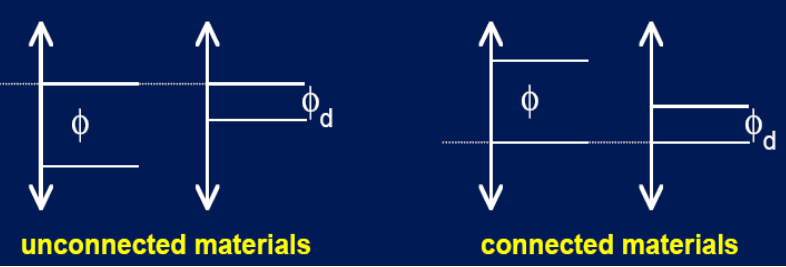
Energy level alignment
Not connected: KE0 levels aligned (same vacuum levels.
Connected: EF levels are aligned.
KE work function relationship
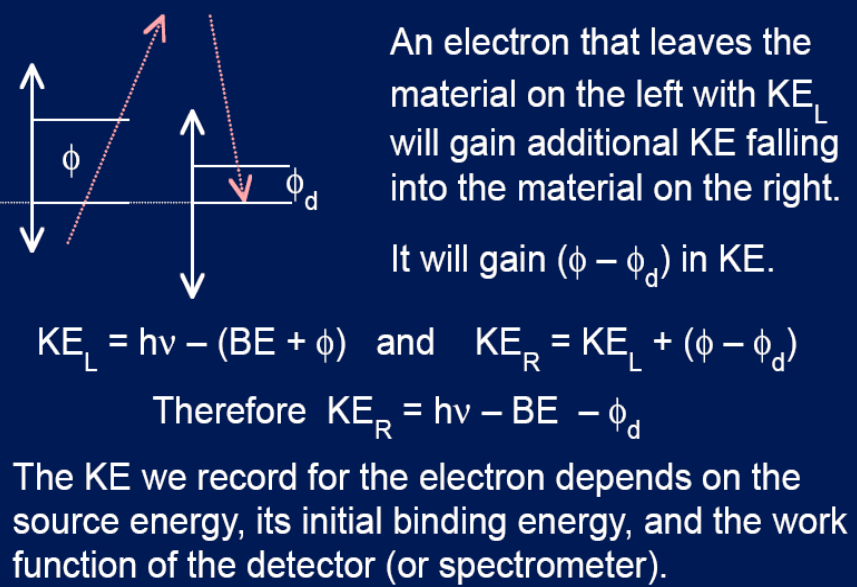
XPS requirements
Sample and detector at same potential
accurate value of energy source
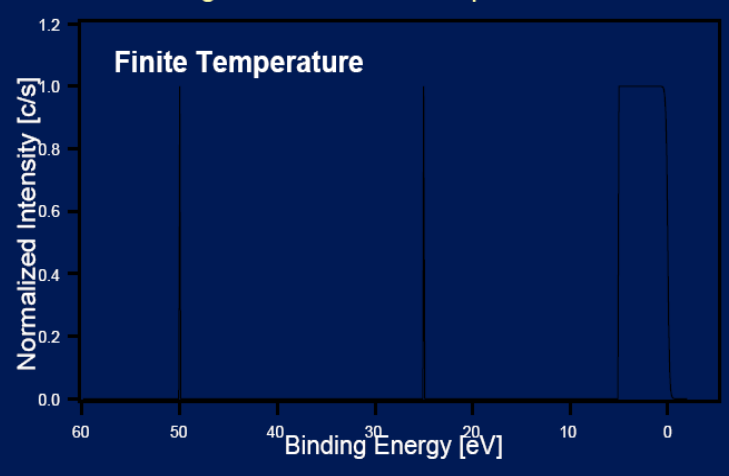
Principles: Fermi distribution (T=0K)
Cause: At temperatures T ≠ 0, the fermi level is smeared out according to the fermi distribution function.
Effect: Smearing of valence band
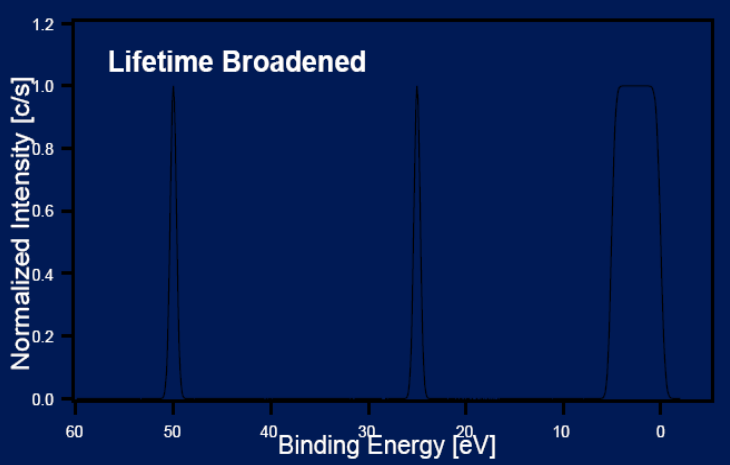
Principles: Uncertainty principle
ΔΕ Δt = h/4π
ΔΕ: energy broadening
Δt: life-time of electron
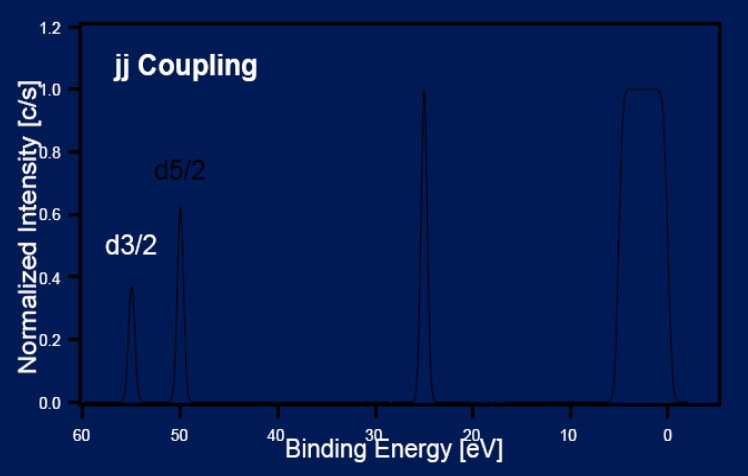
Principles: Electron Spin
Electrons have spin (± 1/2), diffrent spin orbit coupling means diffrent binding energies.
j-j: momenta Z >75
l ± s: sum of total spin and orbit angular momenta - proportinal to ratio of occupancy relative
Principles: Intensity
Efficiency of phton interaction with the electron is dependent on phtoelectron cross section.
Low cross section low intensity.
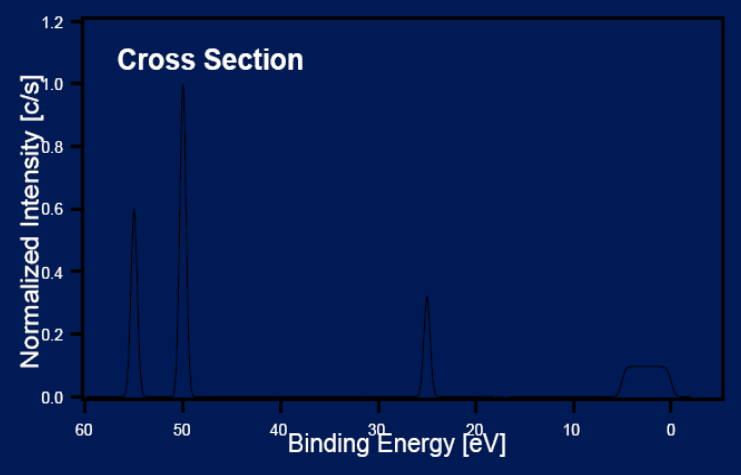
Electron Distribution Curve
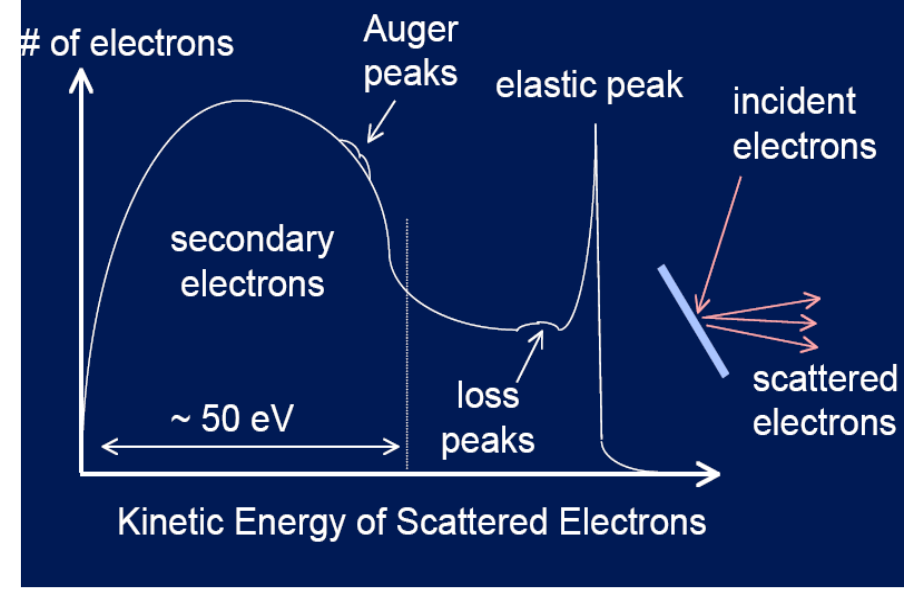
Inelastic Scattering: loss peaks
when the primary electron looses energy due to a single scattering event as it leaves the sample
Inelastic Scattering: secondary electrons
When the primary electron scatters multiple times and causes other (low energy) electrons to be ejected from the material
Principles: Inelastic scattering
Causes an increase in the background level on the high binding energy side of all peaks in an XPS spectrum.
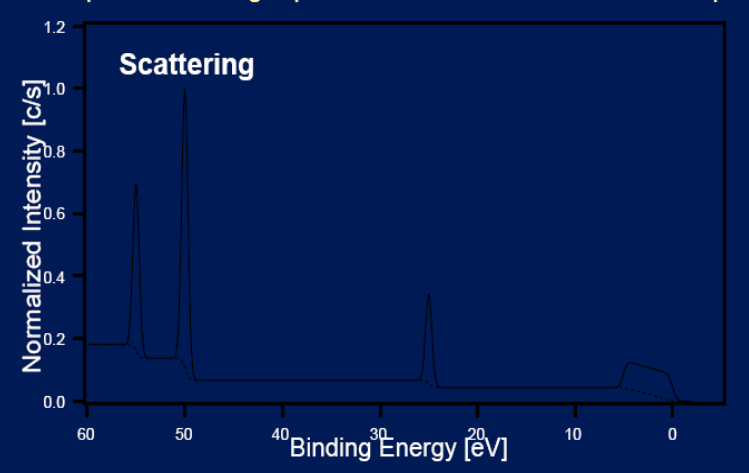
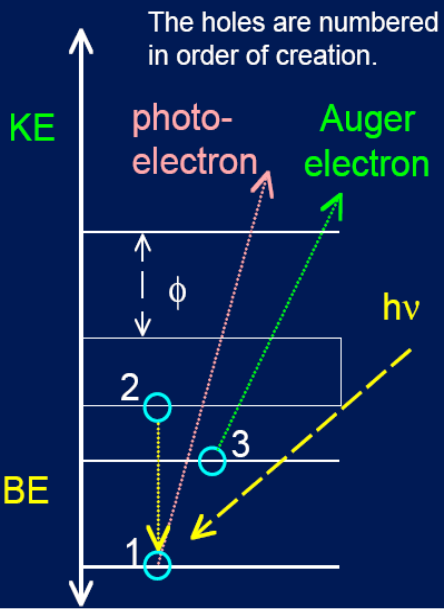
Auger scattering
Create a hole in core level - electron falling ejects auger electron
Principles: X-ray satellites
X-ray sources have finite line widths.
secondary emmision line at lower binding energy to main peak.
Analyzer transmission
Acceptance aperature is finite - line broadening.
Kinetic energy of transmitted electron not all equal - discrepencies in peak heights
Peak Positions
Info:
elements in the material
oxidation state
Error factors:
overlapping peaks
charging
satelites
Peak half widths
Info:
photoemission llifetime
oxidation state
Error factors:
overlapping peaks
source/analyzer broadening
Peak areas
Info:
concentration
Error factors:
overlapping peaks
sampling depth
Chemical State Effects
factors which influence the state charge of an atom before a photon strikes it.

Hybridization
The BE of an electron in agiven orbital changes due to a change in hybrization state of the atom.
Oxidation State
The BE of an electron increases as the oxidation state of the atom increases. Tracks with 1/R
Degree of Bonding
As the atom looses valence electrons, the BE of all remaining electrons increases. Electronegativity/atom sizes/no of neighbouring bonds determine magnitude
Chemical state effects: Magnitude
When an electronic change occurs in an atom, the change in potential felt by an electron in an orbital at R from nucleus goes 1/R.
more significant for core orbitals (small R)
decrease going down the group (increase in R)
Increase across period (decrease in R)
Angle-Dependent XPS
Angle of electron emission is varied, improving depth profiling without destructive sputtering.
Escape Depth and Inelastic Mean Free Path
Electrons emitted from a sample undergo inelastic scattering, limiting their escape depth. The IMFP (λ) is the average distance an electron travels before losing energy.
Angle Dependence
effective escape depth (d) of photoelectrons

Signal Intensity
Detected photoelectron signal diminishes exponentially with depth - analogous to beer lambert law
